Abstract
Determination of monoclonality through an evaluation of immunoglobulin heavy chain (IgH) gene rearrangements is a commonly performed and useful diagnostic assay. Many laboratories that perform this assay do so by the polymerase chain reaction (PCR). To evaluate current methods for performing IgH gene testing, 19 different Association of Molecular Pathology (AMP) member laboratories analyzed 29 blinded B cell and T cell lymphoid neoplasm samples of extracted DNA and formalin-fixed, paraffin-embedded (FFPE) tissue and were asked to complete a technical questionnaire. From this study, it is clear that Southern blot analysis remains the diagnostic gold standard, with a 100% diagnostic sensitivity and specificity. There was, however, remarkable heterogeneity in the performance of, and results obtained from, IgH PCR assays with diagnostic sensitivity ranging from over 90% to as low as 20%, when evaluating the same specimens. Many laboratories overestimate the diagnostic sensitivity of their IgH PCR assay, and there was a significant, and under appreciated, drop-off (from 61.3% to 41.8%) in detection in paired FFPE as compared with fresh/frozen tissues. Fixation has a dramatic impact on the inability to perform the test on FFPE (43.1%) versus DNA already extracted from fresh or frozen tissue (2.8%). A number of variables that affected the outcome of IgH PCR were identified. Strategies that improved the detection of monoclonal IgH rearrangements include: the addition of FRII to the FRIII upstream primer (increasing detection from 57.3% to 73.6%) and the use of the FR3A rather than the FR3 FRIII primer (increasing detection from 54.7% to 69.7%). Although numerous variables (from DNA extraction to PCR product detection) were evaluated, making it difficult to mandate alterations in laboratory practice, these findings ought to prompt diagnostic molecular pathology laboratories to reevaluate their claims of sensitivity, as well as their methodologies. Both pathologists and surgeons need to ensure that not all submitted material is fixed, if there is adequate sample. Importantly, there is a need for greater standardization to reduce the unacceptably high false negative rate of this crucial diagnostic assay.
The analysis of genetic profiles has entered the mainstream of hematopathology practice, and provides information that is germane to contemporary classification of hematological malignancies. 1 Such analyses provide information that is useful both diagnostically as well as prognostically. 2 Given that many of these are polymerase chain reaction (PCR)-based assays, with their exquisite sensitivity, they have an increasingly important role in monitoring minimal residual disease. 3 Accordingly, even in scenarios where molecular studies are not a prerequisite to making a specific diagnosis, they have utility in identifying a tumor-specific marker that subsequently may be used to track disease after therapy. These molecular assays may be broadly divided into those that evaluate pathological rearrangements, reflecting chromosomal translocations, or physiological rearrangements, reflecting antigen receptor gene rearrangements. Additionally, the evaluation of antigen receptor gene rearrangements is a particularly useful tool in helping to distinguish benign reactive lymphoid proliferations from neoplastic lymphoproliferations, especially in scenarios where morphology, histology, and immunophenotypic analysis is equivocal, or difficult to perform.
The majority of lymphoid malignancies encountered in the West are of B cell lineage. Consequently, analysis of immunoglobulin (Ig) gene rearrangements is one of the most frequently ordered molecular hematopathology assays. Of the three Ig genes that rearrange (the heavy chain gene, and the kappa and lambda light chain genes), the heavy chain (IgH) gene is the one that is most frequently studied. The major reason for this is physiological: the IgH gene rearranges before the light chain genes and some B cell malignancies, typically precursor lymphoblastic leukemias and lymphomas, have not yet rearranged their light chain genes. 4 Indeed, Southern blot analysis (SBA) of the IgH gene was one of the first molecular genetic tools used in the diagnostic scenario. 5, 6 However, given the numerous limitations associated with SBA, over the last few years it has been supplanted by PCR analysis of this locus. 7
PCR analysis of the IgH gene typically involves the use of a consensus primer pair, with the upstream primer being homologous to a V segment and the downstream primer annealing to one of the J segments. 8 Usually, a single J region primer is sufficient to recognize all six possible J segments, but no single V region primer recognizes all V segments, since there are many more V segments and they are more heterogeneous, as compared with J segments. This is the primary explanation for the lack of a 100% diagnostic sensitivity of single primer pair IgH PCR assays. In a non-B cell, the V and J segments are too far apart (∼ 70 kb) to be amplified by PCR. 9 When a B cell rearranges its IgH V, D, and J segments, the V and J segments become sufficiently close to be PCR amplifiable. A monoclonal IgH gene rearrangement is primarily distinguished from polyclonal rearrangements based on the homogeneity of size of the amplified fragment in the former, versus the heterogeneity of the differently sized fragments in the latter. This difference in size is largely dependent on the number of N sequences added by the intra-nuclear enzyme, terminal deoxynucleotidyl transferase (TdT), at the time of V-(N)-D-(N)-J rearrangement.
Although immunophenotypic analysis of unfixed material evaluating Ig light chain restriction is the simplest method of determining the monoclonality of mature B cell lymphoproliferations, such studies are frequently unreliable in fixed tissues. Furthermore, fixed tissue is not amenable to SBA. There has also been a movement toward making diagnoses by less invasive procedures on smaller and smaller pieces of material (ie, needle biopsy, fine needle aspirate), which may not always be suitable for comprehensive immunophenotypic analysis or SBA. 10, 11 Accordingly, IgH PCR has assumed an increasingly important role in the diagnostic armamentarium. However, there are many factors, both pre-analytic and analytic that affect the result and validity of an IgH PCR assay. These include the specific subtype of B cell lymphoma being evaluated, whether the diagnostic material is fresh/frozen or fixed, as well as numerous variables inherent to the PCR procedure. 12, 13
Based on this, the aims of this study were to: gauge the inter-laboratory heterogeneity in the performance of IgH PCR; evaluate the utility and methodology used in laboratories performing IgH PCR analyses; determine the effects of fixation; and identify those variables that may affect the outcome of such analyses, so that certain parameters may be altered to optimize detection rates. To this end, the authors circulated a total of 29 blinded samples from B and T cell lymphomas of DNA already extracted from frozen tissue (n = 16) and paraffin-embedded tissue (n = 13) to 30 clinical diagnostic laboratories. Results were obtained from nineteen laboratories, and provide the basis of this report.
Materials and Methods
Members of AMP were surveyed through an electronic listserv announcement for their interest in participating in a sample exchange to evaluate IgH gene rearrangement testing. This was undertaken in conjunction with a similar evaluation of T cell receptor gene rearrangement testing. 14 Thirty respondents were sent 29 blinded samples (16 DNA samples and 13 samples of sections of formalin-fixed, paraffin-embedded tissue (FFPE)) and technical questionnaires. The participating laboratories were given no clinical or pathological information pertaining to these samples.
Frozen cells and available corresponding paraffin-embedded tissue of archived lymphoma samples originally diagnosed using the Revised European-American Classification of Lymphoid Neoplasms (REAL) classification system 15 at the Oregon Health Sciences University Department of Pathology were retrieved for use in this study.
All of the cases had been evaluated by SBA of high molecular weight (HMW) DNA. A monoclonal rearrangement was documented by the presence of one or two non-germline bands in two or more restriction enzyme digestions. Monoclonal IgH gene rearrangements, using a JH probe, were detected in all B cell cases, and monoclonal T cell receptor β-chain (TCRβ) gene rearrangements, using Jβ1 and Jβ2 probes, were detected in all of the T cell cases. By contrast, none of the B cell cases harbored monoclonal TCRβ gene rearrangements and none of the T cell cases harbored monoclonal IgH gene rearrangements, when analyzed by SBA of HMW DNA.
A summary of the distributed specimens is presented in Table 1 . Extracted DNA from 16 samples was aliquoted and distributed to each participating laboratory. DNA extraction was performed from frozen cells as previously described. 14 DNA concentration was determined and dilutions were made with Tris-EDTA to a final concentration of 0.1 μg/μl. Amounts of 500 μl (50 μg of DNA) or 100 μl (10 μg of DNA) were aliquoted for distribution to the participating laboratories.
Table 1.
Specimens Distributed for the Sample Exchange
| Specimen | No. of specimens | DNA | Paraffin | Paired |
|---|---|---|---|---|
| B cell | ||||
| FL | 11 | 7 | 4 | 4 |
| SLL/CLL | 3 | 2 | 1 | 1 |
| MCL | 1 | 0 | 1 | 0 |
| Total B cell specimens | 15 | 9 | 6 | 5 |
| T cell | ||||
| PTCL | 9 | 4 | 5 | 4 |
| CTCL | 2 | 1 | 1 | 1 |
| TLBL | 3 | 2 | 1 | 1 |
| Total T cell specimens | 14 | 7 | 7 | 6 |
| Total all specimens | 29 | 16 | 13 | 11 |
FL, follicular lymphoma; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukemia; MCL, mantle cell lymphoma; PTCL, peripheral T cell lymphoma; CTCL, cutaneous T cell lymphoma; TLBL, T cell lymphoblastic lymphoma.
Samples from 13 paraffin-embedded specimens were also distributed, 11 of which corresponded to frozen cell specimens. Participants were not informed that there were paired samples. For paraffin-embedded tissues, four or five 10-μ sections of each block were cut and distributed, as curls in tubes, to each participating laboratory. DNA extraction was performed in the individual laboratories, using their standard clinical procedures. Similarly, laboratories were asked to follow their validated protocols for PCR testing.
The distributed samples were accompanied by a technical questionnaire, addressing specifics regarding IgH PCR and SBA test performance. Laboratories were requested to return the completed questionnaire, a summary of results and copies of diagnostic radiographs, and/or gels used for interpreting results.
χ2 analysis of probability was performed using two web-based resources. Probability (P) values of 0.05 or less were considered statistically significant.
Results
IgH PCR results were obtained from 19 laboratories and survey results were obtained from 17 laboratories (all of which also provided IgH PCR results). In some instances, there were incomplete responses with regard to the completion of all analyses and the survey itself. Fourteen laboratories performed SBA for IgH gene rearrangements on some or all of the extracted DNA specimens.
Technical Questionnaire
Southern Blot Analysis
Most laboratories (13 of 17, 76%) used inorganic methods to extract DNA from fresh or frozen material, with the remainder using organic extraction methods. Regarding restriction enzymes, 7 of 16 (44%) used the standard restriction endonucleases BamHI, EcoRI, and HindIII, while 9 of 16 (56%) often added BglII and/or XbaI. Of the 16 laboratories responding, 12 (75%) required two or more enzymes to display rearranged bands for a positive result, with 4 (25%) interpreting a non-germline band with a single enzyme as being sufficient to document a case as being rearranged. Eight laboratories (57%) used isotopic probes and 6 (43%) used non-isotopic methods to label their probes.
PCR Analysis
Paraffin section extraction methods varied from inorganic in five (29%), organic in four (24%), to crude lysate in four (24%) laboratories. No information was provided by four (24%) laboratories. Eight centers (47%) used a hot start PCR approach while nine (53%) did not. Eight (47%) of the responding laboratories used a single, non-nested PCR method, six (35%) a semi-nested approach while one (6%) used nested primers, with two not providing this information. Eight laboratories (47%) used only a third framework region (FRIII) for the V primer, with seven (41%) also using an FRII primer, and one (6%) adding an FRI primer. One respondent did not report which upstream primer(s) they used. With regard to which specific FRIII primer was used, 11 of 17 (65%) centers used a sequence identical or very similar to the FR3A primer 16 while 6 of 17 (35%) used a primer identical or very similar to that designated FR3, which has an extra six 5′ bases as compared with the FR3A primer. 17, 18 This primer has also been designated Vcon. 19 In general, while one of two FRIII primers was used, as noted above, four different downstream JH primers were used. Seven laboratories (41%) used a primer identical or very similar to JHa, 20 while six (35%) used a primer similar to LJH, 16 which has three fewer 5′ bases than the JHa primer. In two laboratories (12%), a primer with an extra five 3′ bases was used which is similar or identical to that designated CFW1 21 or JH1245. 22 Two laboratories (12%) used JH primers that appear to have a variant sequence at the 5′ end. 8, 17 A DNA sequence corresponding to this anti-sense primer could not be identified on a BLAST search.
PCR products were analyzed on polyacrylamide gels in nine laboratories (53%), by capillary electrophoresis in four (24%), on agarose gels in three (18%), and on MetaPhor gels (BioWhittaker Molecular Applications, Rockland, ME) in one laboratory (6%). Visualization of the PCR product in gels was most often performed by ethidium bromide (81%), followed by Sybr Green (Molecular Probes, Eugene OR) in 13%, and silver staining in 6% of laboratories. Positive controls included patient samples (59%) and cell lines (35%), including Raji, SUDHL5, IM9, and SUPB15, with Raji used most often. The type of control was not reported for one laboratory (6%). Regarding sensitivity controls, diluted DNA was used by 59%, diluted cells by 21%, and none by 20% of respondents. The range of sensitivities predicted by the laboratories was 0.001% to 10% for both frozen and paraffin samples, with a median of 1% for the former and 1% to 5% for the latter. Laboratories predicted that their IgH PCR methodology could detect 57% to 94% of IgH gene rearrangements detectable by SBA, with a mean of 77%.
Sample Exchange Results
Southern Blot Analysis
None of the 14 laboratories that performed SBA did so on every specimen, presumably due to a lack of sufficient amount of DNA for all tests in the sample exchange. However, all of the cases of B cell lymphoma tested (75 of 75, out of a possible total of 98 = 14 laboratories × 7 DNA specimens) were positive by SBA. Similarly, all of the cases of T cell lymphoma tested (67 of 67, out of a possible total of 98) were negative by SBA. Since all of the laboratories scored perfectly in the SBAs performed, it is clearly not possible to evaluate any of the differences in methodology, as gleaned from the technical questionnaire.
PCR Analysis: B Cell Lymphomas
Nineteen laboratories reported results for IgH PCR in the 15 samples of B cell lymphoma. Only 5 of these 19 laboratories reported results on all 15 specimens. Positive IgH PCR results were obtained in 147 of 232 assays (out of a possible total of 285 = 19 laboratories × 15 B cell samples), for an overall rate of detection of 63.4%, as detailed in Table 2 . There was a significant decrease in the ability to detect a monoclonal IgH gene rearrangement in the follicular lymphomas (55.2%) as compared with the other lymphomas (83.6%), P < 0.0001.
Table 2.
Overall Results of IgH PCR
| B cell lymphomas* | T cell lymphomas | |
|---|---|---|
| Positive result | 147 /232 (63.4%) | 13 /208 (6.3%) |
| Follicular lymphomas | 91 /165 (55.2%) | |
| Non-follicular lymphomas | 56 /67 (83.6%) |
Data reflect all samples analyzed, both DNA specimens and paraffin-embedded tissues. Denominators are the total numbers of specimens successfully tested. All B cell lymphomas were positive by IgH SBA, while all T cell lymphomas were negative by IgH SBA.
No single laboratory scored 100% on all B cell lymphoma samples. The best performing laboratory scored 92% while the worst performing scored 20%.
11 of the 19 laboratories scored a perfect 0% false positive rate.
P < 0.0001. Unlike this disease category difference in the B cell lymphomas, no such stratification was noted among the types of T cell lymphoma.
PCR Analysis: T Cell Lymphomas
Nineteen laboratories reported results for IgH PCR in the 14 samples of T cell lymphoma. Only 5 of these laboratories reported results on all 14 specimens, with 3 of these 5 being among the 5 that were able to evaluate all of the B cell lymphomas noted above. Positive IgH PCR results were obtained in 13 of 208 assays (out of a possible total of 266 = 19 laboratories × 14 samples). Thus overall, IgH PCR was positive in 6.3% of all T cell lymphoma specimens tested (Table 2) . Eleven of nineteen laboratories obtained a “perfect score” of 0%, with 2 of the other 8 laboratories having rather high false positive rates (3 of 11 for a rate of 27.3% in one laboratory and 2 of 8 for a rate of 25% in another). The false positivity rate ranged from 1 of 19 (5.3%) to 2 of 16 (12.5%) for any one case of T cell lymphoma, but no statistically significant specific disease association was evident. Although there were two laboratories with a greater than or equal to 25% false positivity rate, the one with the higher (27.3%) rate was one of the two laboratories that did not respond to the technical questionnaire. The other was the only laboratory that reported using FRI primers (see below).
PCR Analysis: Effects of Fixation
There was a significant decrease in the ability to detect IgH gene rearrangements in B cell lymphomas which were FFPE as compared with the DNA specimens (P = 0.012) (Table 3) . This ranged from 44.4% in the worst-performing to 100% in the best-performing laboratory, with a mean of 61.3% (57 of 93) for extracted DNA samples, while for FFPE samples this ranged from 11.1% in the worst-performing to 66.7% in the best-performing laboratory, with a mean of 41.8% (22 of 55). Twenty-three percent of samples overall (96 of 418), were not tested, presumably due to the lack of amplification of an internal control gene. This lack of testing was significantly higher for the paraffin-embedded samples (Table 3) .
Table 3.
Effect of Fixation
| DNA | FFPE | P value | |
|---|---|---|---|
| B cell lymphomas | 57 /93 (61.3%) | 22 /55 (41.8%) | 0.012 |
| T cell lymphomas | 4 /110 (3.6%) | 7 /64 (10.6%) | 0.056 |
| Non-reportable results | 6 /209 (2.8%) | 90 /209 (43.1%) | <0.0001 |
Data pertain to the 11 paired samples only (5 B cell, 6 T cell, with results obtained from 19 laboratories, thus 11 × 19 = 209 possible results). Fractions and percentages reflect the detection of a monoclonal IgH gene rearrangement. DNA, DNA extracted from fresh or frozen tissue; FFPE, formalin-fixed, paraffin-embedded tissue, DNA extracted in individual laboratories.
PCR Analysis: Effects of Differences in Methodology
The IgH PCR results were further analyzed at the level of seven technological variables. These were: paraffin extraction methods; PCR method (hot start versus standard); the use of nested primers (single versus semi-nested versus nested); the use of upstream V primers (FRIII alone, or with FRII or FRI); the specific type of V-FRIII primer used (two); the specific J primer used (four); as well as the final PCR product detection method used (polyacrylamide gel electrophoresis versus capillary electrophoresis versus agarose gel electrophoresis versus MetaPhor gel electrophoresis).
The three extraction methods used for paraffin embedded tissue had no statistically discernible effect on the ability to detect a monoclonal IgH gene rearrangement in the B cell lymphomas (Table 4) . However, organic extraction was associated with more false positives (14%) than the other two methods (P = 0.04). No significant differences in false negativity or positivity rates were observed when hot start was compared with standard PCR (P = 0.4 and 0.26, respectively, data not shown). Similarly, there was no impact on outcome (both sensitivity and specificity) when a single primer pair was compared with a semi-nested or nested approach (P = 0.12 and 0.84, respectively, data not shown).
Table 4.
Effect of Methods of DNA Extraction from Paraffin-Embedded Tissue
| Extraction method | Number of laboratories (%) | True positives (%) | False positives (%) |
|---|---|---|---|
| Organic | 4/17 (24%) | 15/21 (71%) | 3/21 (14%)* |
| Inorganic | 5/17 (29%) | 12/24 (50%) | 0/27 (0%)* |
| Crude lysate | 4/17 (24%) | 6/13 (46%) | 0/16 (0%)* |
| Not stated | 4/17 (24%) | 3/7 (43%) | 0/4 (0%) |
| Totals | 17/17 (100%) | 36/55 (55%) | 3/68 (4%) |
Fractions reflect total number of tests positive/total number of tests performed by the participating laboratories, on all distributed formalin-fixed paraffin-embedded tissue material (paired and unpaired). The total number of fixed tissue specimens distributed, to laboratories that responded (n = 17), was 221 (13 × 17). True positives, finding a monoclonal IgH gene rearrangement by PCR in B cell lymphomas; false positives, finding a monoclonal IgH gene rearrangement by PCR in T cell lymphomas.
The only significant difference (P = 0.04) was noted here, with a greater frequency of false positives when using organic extraction methods.
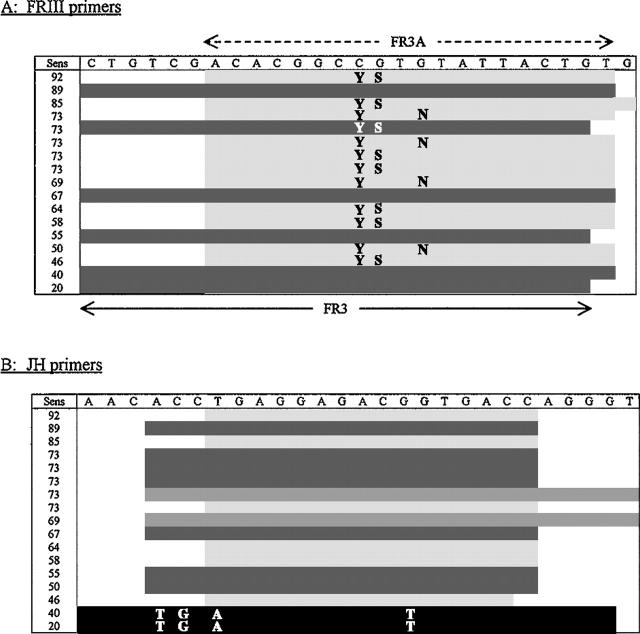
When the use of FRIII, FRII, and FRI were compared with one another, there was no significant difference in the detection of IgH monoclones in the B cell cases. However, when FRIII alone was compared with the added use of FRII, a significant difference was observed (P = 0.021) (Table 5) . When evaluating the effect of V primer use on the false positivity rate, this was greatest when using FRI, notwithstanding the fact that the numbers in the FRI group are low. Furthermore, the specific FRIII primer used affected the IgH PCR positivity rate in the B cell lymphomas but not in the T cell lymphomas. In the B cell lymphomas, use of the FR3A primer resulted in a positive outcome in 69.7% (92 of 132), while the FR3 primer was associated with a 54.7% (41 of 75) detection rate (P = 0.03) (Figure 1) . Although there were more false positives with the use of FR3A (8 of 117, 6.8%) as compared with FR3 (1 of 67, 1.5%), this was not statistically significant (P = 0.11). There was no difference in outcome in the B cell lymphomas based on which of the three legitimate JH primer were used (JHa, LJH, or CFW1), while the use of the “variant” primer was, not surprisingly, associated with a significant diminution in the positivity rate (P < 0.0001). However, there was a significantly lower false positivity rate with JHa (0 of 71, 0%) as compared with LJH (6 of 61, 9.8%) and CFW1 (2 of 24, 8.3%), P = 0.028. The PCR product detection method (polyacrylamide gel, agarose gel, MetaPhor gel, or capillary electrophoresis) had no impact on false positive or false negative rates (P = 0.61 and 0.40, respectively, data not shown).
Table 5.
Effect of Using Additional FR Primers
| FR primer | Number of laboratories (%) | True positives (%) | False positives (%) |
|---|---|---|---|
| FRIII only | 8/17 (47%) | 55/96 (57.3%)* | 6/91 (6.6%)†‡ |
| + FRII | 7/17 (41%) | 64/87 (73.6%)* | 1/75 (1.3%)†‡ |
| + FRI | 1/17 (6%) | 8/11 (72.2%) | 2/8 (25%) |
| Not stated | 1/17 (6%) | 6/13 (46.2%) | 0/10 (0%) |
| Totals | 17/17 (100%) | 147/232 (63.4%) | 13/208 (6.3%) |
Fractions reflect total number of tests positive/total number of tests performed by the participating laboratories, on all distributed material (DNA and paraffin-embedded). True positives, finding a monoclonal IgH gene rearrangement by PCR in B cell lymphomas; False positives, finding a monoclonal IgH gene rearrangement by PCR in T cell lymphomas.
P = 0.021.
P = 0.09.
P = 0.011.
Figure 1.
Immunoglobulin heavy chain gene primers. Sequences (5′→3′) of IgH primers used by the individual laboratories, ranked according to ability to detect a monoclonal IgH rearrangement in the distributed B cell lymphoma specimens. Sens; sensitivity, expressed as a percentage detection for each individual laboratory. A: FRIII primers. As compared with FR3A, FR3 has an extra six 5′ bases and one less 3′ base. The letters in the body of the table reflect nucleotide wobble in the primer sequence (Y, C/T; S, G/C; N, G/A). = identical or similar to FR3A sequence (average detection rate, 69.7%). = identical or similar to FR3 sequence (average detection rate, 54.7%). This difference in detection rate is significant (P = 0.03). B: JH primers. The letters in the body of the table reflect the variation in the primer nucleotide sequence, as compared with that designated at the top. = identical or similar to JHa sequence (average detection rate, 70.4%). = identical or similar to LJH sequence (average detection rate, 69.4%). = identical or similar to CFW1 sequence (average detection rate, 70.8%). = variant sequence (average detection rate, 30%). This difference in detection rate for the variant sequence is significant (P < 0.0001).
Discussion
The primary objective of this study was to compare methodologies across laboratories (inter-laboratory), rather than to evaluate different methodologies in a single laboratory (intra-laboratory), to identify variables affecting the outcome of IgH gene PCR analysis. This study identified or confirmed a number of factors that may be of practical value to laboratories performing IgH gene analysis. It should be noted, however, that given the design of the study, with the complexity of assay performance, and that a multitude of variables may impact on the outcome, it is somewhat of a challenge to draw unequivocal conclusions, and make definitive recommendations, from this analysis. Since the protocols for performing this assay are quite heterogeneous in the different clinical diagnostic laboratories participating in this study, it is not possible to investigate each parameter separately and independently. However, certain differences in the outcome of this assay can be attributed to some technical variations that were identified.
Southern blot analysis (SBA) remains the gold standard, and proved itself to be the most sensitive and specific diagnostic test for identifying IgH gene rearrangements in the current study. One hundred percent of B cell lymphomas evaluated were positive by SBA, and 100% of T cell lymphomas were negative by SBA. These results were obtained despite the fact that 25% of responding laboratories indicated that a non-germline band with only a single restriction enzyme was sufficient to document a case as being truly rearranged. This latter practice is contrary to the National Committee for Clinical Laboratory Standards (NCCLS) recommendations. 23 Nevertheless, when sufficient fresh or frozen tissue is available, SBA cannot be superceded as a robust and stringent test for the diagnostic detection of monoclonal IgH gene rearrangements. However, even when such tissue is available, it is not always logistically feasible to perform SBA, given all of the features that identify PCR as a more practical test for most diagnostic laboratories (including faster turn-around time, lower cost, and absence of reliance on radioactive materials).
Despite the fact that one of the advantages of PCR over SBA is the ability to perform the analysis on small pieces of FFPE tissue, the degree to which fixation negatively impacts the validity of the assay is perhaps under appreciated. Indeed, this was evident at two levels in our study. First, when comparing the 11 paired specimens, there was a significant diminution in the diagnostic sensitivity of the assay with IgH PCR being positive in 41.8% of FFPE tissue as compared with 61.3% of paired extracted DNA, P = 0.012. Second, PCR was much less likely to be performed on FFPE tissue (43.1%) versus DNA already extracted from fresh or frozen tissue (2.8%), P < 0.0001. This difference was presumably due to the absence of amplifiable DNA in the former. However, this was not formally addressed in the survey or technical questionnaire, in that we did not specifically ask whether an internal control for DNA integrity/amplifiability was performed.
Evaluating monoclonal IgH gene rearrangements by PCR has numerous limitations, the most relevant of which is the well recognized false negativity rate. Numerous mechanisms account for this, the most relevant being the use of consensus V primers that do not recognize all possible V segments. An additional mechanism is the deletion of 3′ bases in the V segments at the time of V-D-J recombination. 24 The participants surveyed in this study were well aware of the overall false negativity rate of IgH PCR, with a predicted mean of approximately 23% of IgH PCR assays missing an IgH monoclone. The results of the sample exchange, however, indicate that these laboratories fall somewhat short of this prediction of 23%, with an even greater documented false negativity rate of 37.5% overall. In fact, as noted in the paired specimen analysis, greater than 58% of FFPE B cell lymphoma tissues were IgH PCR negative. These data thus suggest that a number of clinical diagnostic laboratories have neither necessarily formally validated the performance of their IgH PCR assay, nor fully appreciated the significantly high false negativity rate in studies performed on FFPE tissues.
The significant diminution in IgH PCR positivity in FFPE tissues noted in this inter-laboratory analysis is in contrast to what has been published by others. Indeed, in one series, there was only an 8% false negativity rate in FFPE tissues. 25 Furthermore, three additional studies did not find decreased diagnostic sensitivity when comparing fixed with fresh tissues. 22, 26, 27 While it is now well recognized that the use of B5 and Bouin’s fixatives impact negatively on the ability to perform PCR, 28, 29, 30 it is not the explanation for the discrepancy in this study, since all specimens were formalin-fixed. Individual laboratories may be reporting higher success rates based on their ability to control fixation at their particular institution. However, this is likely to remain an issue for laboratories that perform reference and consultation work.
A valid explanation for this higher than expected false negativity rate in our study is likely the disproportionate representation of follicular lymphomas, which accounted for 11 of the 15 B cell lymphoma specimens that were circulated. Follicular lymphomas are well recognized to be associated with a lower yield of positive IgH PCR assays, 18, 21, 22, 31 and this was confirmed by us. Even with the relatively low numbers of B cell lymphomas other than those of follicle center cell origin in this study (n = 4), there was a significant difference in the ability to detect a monoclonal IgH gene rearrangement by PCR. A positive result was detected in only 55.2% of follicular lymphomas, as compared with 83.6% for the other lymphomas (P < 0.0001). This low yield parallels that reported by others, when using a similar simple single PCR reaction with consensus FRIII and JH primers. 13, 20, 32
A number of factors probably account for the higher false negativity rate in follicular lymphomas. Somatic hypermutation of the IgH gene, a consequence of the neoplastic cell having traversed the germinal center, is likely the major explanation for the higher rate. 33 Additionally, the inability to detect a B cell monoclone by PCR may be compounded by the presence of increased numbers of diluting polyclonal B cells, whose IgH genes would compete for the primers. 27 Another putative explanation is that follicular lymphomas are somewhat different from most other common B cell lymphomas in that one of the IgH loci potentially amenable to IgH PCR is rendered inaccessible, as a consequence of the presence of a t(14;18) translocation, in a significant majority of these lymphomas.
Such mechanisms notwithstanding, there is a clear need to employ alternative strategies to document monoclonality in follicular lymphomas, especially to identify a tumor-specific fingerprint that is prognostically important to use to measure minimal residual disease. The inclusion of primers to detect the t(14;18) translocation is a logical addition, and the diagnostic yield is improved with this. 20 The use of FRII primers has also been shown to reduce the false negativity rate both generally, as well as specifically in follicular lymphoma. 32 Indeed, our study confirmed the value of adding an FRII primer to the use of FRIII alone, increasing the diagnostic sensitivity from 57.3% to 73.6% (P = 0.021).
The addition of FRII primers has been shown to be advantageous across the spectrum of lymphomas. 13, 34 Some have suggested that the inclusion of FRIII does not increase the detection of IgH PCR positive cases, 31 and that FRII and FRI primers alone may suffice. However, as the analysis progresses from FRIII, to FRII and FRI, the size of the amplicon increases as well. While the amplification of FRIII and FRII, with product sizes of approximately 70 to 130 bp and 220 to 280 bp, respectively, is usually feasible for FFPE tissues, this may not be the case with FRI primers, where the amplicon is typically 300 to 320 bp in size. Some laboratories, however, have successfully amplified the latter from FFPE tissue. 35 Additional strategies that have been proposed to reduce the frequency of false negatives include the use of semi-nested PCR, 36 the use of radiolabeled nucleotides, single stranded conformational polymorphism (SSCP), heteroduplex analysis, blotting and probing the PCR products, as well as evaluating immunoglobulin light chains by PCR. 37 In the current analysis, we did not observe an increased sensitivity, nor an increase in false positives, in laboratories performing semi-nested PCR. However, we would strongly caution against the use of semi-nesting, given the significant hazard of contamination. Furthermore, some of the other approaches are likely too labor intensive and/or have not yet been formally evaluated for routine diagnostic utility.
Although many others have shown the added value of including FRII primers, this study is the first to show the advantages and disadvantages of specific V (FRIII) and J primers. With regard to FRIII primers, the use of FR3A as compared with FR3 was associated with an increase in the diagnostic sensitivity from 54.7% to 69.7% (P = 0.03). However, it is notable that one laboratory (Figure 1) had an 89% detection rate with the FR3 primer, clearly highlighting that more variables than primer sequence affect the final outcome. Others have also noted the superiority of FR3A over FR3; 18 however, that study only demonstrated this in the context of a semi-nested reaction, not as a single primer. Clearly too, laboratories need to be particularly careful to check the sequence of their primers. The two laboratories that used a “variant” JH primer have significantly dismal (but not completely negative, likely due to the variance being at the 5′, rather than 3′, end of the primer) detection rates of 20% and 40%, respectively. The use of the JHa primer was shown to have the lowest false positivity rate, as compared with the LJH and CFW1 primers. This is in contrast to what has been alluded to previously, namely that the longer J1245 primer (identical to CFW1) has a higher specificity. 38 Another study noted that the use of this primer is associated with a lower sensitivity, 22 although we were not able to confirm this finding either. While the current survey demonstrated a higher IgH false positivity rate for FFPE cases extracted by organic methods, the tandem T cell receptor survey showed more T cell receptor-γ false positives with inorganic extraction methods. 14 The explanation for this difference is unclear.
The issue of finding monoclonal IgH rearrangements in unequivocal, bona fide T cell lymphomas warrants clarification. While such “cross-lineage” rearrangements are well documented, 39 given the absence of finding IgH monoclones by SBA, we interpret this to reflect an analytic sensitivity issue. Whether this is a biological phenomenon, due to the presence of low numbers of autoreactive tumor-specific B cells, or a technical phenomenon, is unclear. With regard to the latter, it is now well established that low numbers of B cells (as may be the case in T cell lymphomas) may be associated with “pseudoclonality.” 40, 41, 42 As noted in this study, some primers, both V and J, may be associated with a higher false positivity rate. On a simpler but nevertheless pertinent practical level, the finding of such rearrangements highlights the need to interpret molecular studies in the appropriate histopathological and immunophenotypic context, and not misdiagnose lineage on PCR data alone.
Based on the heterogeneity of methodologies and outcomes uncovered by this study, there is a clear need to develop some universal standards. The development of standardized technology would be important not only for the diagnosis per se, but also to identify a robust and reliable fingerprint of the B cell clone, to be used to track disease after therapy. The diagnostic utility should not be understated, however, since it is crucial to obtain reproducible and consistent results between testing centers. This study, and others, has identified variables impacting outcome. Other such multi-center studies have been undertaken. For example, in an evaluation of the PCR analysis of t(14;18) translocation, widely disparate results were observed. 43 However, this study evaluated artificial, spiked samples whereas the current study evaluated bona fide clinical specimens. By contrast, a German-Austrian-Swiss multi-center assessment of IgH and T cell receptor-γ performance found no significant differences in results when different individual techniques were compared. 44 There have also been other reports of inter-laboratory surveys evaluating molecular hematopathology assays. 45, 46 However, these reports, assessing BCR-ABL and PML-RARα respectively by RT-PCR, were quite different from the current study, in that they evaluated “artificial” samples (various concentrations of plasmids, and dilutions of cell line DNA or cells, measuring analytic, not diagnostic, sensitivity) rather than “real” clinical specimens. We should aim to emulate the efforts of the BIOMED-1 Concerted Action, which has made significant strides in standardizing the performance of RT-PCR assays of translocations seen in acute leukemia. 47
In summary, this multi-center study generated a large amount of data regarding the performance of IgH PCR. Given the multitude of variables, it is rather difficult to draw definitive conclusions and make rigid recommendations. However, a number of key observations were made: there is remarkable inter-laboratory heterogeneity with regard to diagnostic sensitivity, ranging from over 90% to as low as 20%, when evaluating the same specimens; many laboratories appear to be overestimating the diagnostic sensitivity of their IgH PCR assay; there is a significant, and under appreciated, drop-off (from 61.3% to 41.8%) in detection in FFPE as compared with fresh/frozen tissues; fixation has a dramatic impact on the inability to perform the test, presumably due to absence of amplifiable DNA. For FFPE tissue, this inability to perform the test was evident for 43.1% of specimens, versus only 2.8% for DNA already extracted from fresh or frozen tissue.
Recommendations and strategies that would improve the detection of monoclonal IgH rearrangements include: verification that primer sequences are correct; the addition of FRII to the FRIII upstream primer (increasing detection from 57.3% to 73.6%); the use of the FR3A rather than the FR3 FRIII primer (increasing detection from 54.7% to 69.7%); avoidance of placing the whole specimen in fixative, if there is adequate sample; comparison of fresh/frozen tissue with FFPE success rates within one’s own laboratory; and development of methods to increase the yield of amplifiable DNA from FFPE tissue.
These findings should prompt diagnostic molecular pathology laboratories to reevaluate their claims of sensitivity, as well as methodologies. Importantly, we need to formally recommend strategies to reduce the unacceptably high false negative rate of this crucial diagnostic assay, through the introduction of standardized methodologies.
Figure .
Figure .
Figure .
Figure .
Figure .
Figure .
Acknowledgments
We thank the following participating AMP laboratories: Armed Forces Institute of Pathology, Washington, DC; City of Hope National Medical Center, Duarte, CA; Evanston Hospital, Northwestern University, Evanston, IL; Georgetown University Medical Center, Washington, DC; Johns Hopkins University Hospital, Baltimore, MD; Loyola University Medical Center, Maywood, IL; Marshfield Laboratories, Marshfield, WI; Mayo Clinic, Rochester, MN; Rush Medical Center, Chicago, IL; St. Barnabas Medical Center, Livingston, NJ; SUNY Hospital of Stony Brook, Stony Brook, NY; SUNY University Hospital of Syracuse, Syracuse, NY; University of Florida, Gainesville, FL; University of Missouri, Columbia, MO; University of Texas Health Science Center, San Antonio, TX; University of Texas Medical Branch, Galveston, TX; University of Texas Southwestern, Dallas, TX; William Beaumont Hospital, Royal Oak, MI; and University of New Mexico Health Sciences Center, Albuquerque, NM.
Address reprint requests to Adam Bagg, M.D., Department of Pathology and Laboratory Medicine, 7.103 Founders Pavilion, Hospital of the University of Pennsylvania, 3400 Spruce St., Philadelphia, PA 19104-4283. E-mail: adambagg@mail.med.upenn.edu.
Footnotes
The planning of this study originated in the Hematopathology Subdivision of AMP. The results were preliminarily discussed at the Annual Meeting of the Association of Molecular Pathology in St. Louis, MO in November, 1999.
References
- 1.Jaffe ES, Harris NL, Stein H, Vardiman JW: WHO Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. 2001. France, IARC Press, Lyon
- 2.Bagg A, Kallakury BV: Molecular pathology of leukemia and lymphoma. Am J Clin Pathol 1999, 112:S76-S92 [PubMed] [Google Scholar]
- 3.Bagg A: Minimal residual disease: how low do we go? Mol Diagn 2001, 6:1-7 [DOI] [PubMed] [Google Scholar]
- 4.van Dongen JJM, Wolvers-Tettero ILM: Analysis of immunoglobulin and T cell receptor genes. Part II: possibilities and limitations in the diagnosis and management of lymphoproliferative diseases and related disorders. Clin Chim Acta 1991, 198:93-174 [DOI] [PubMed] [Google Scholar]
- 5.Waldmann TA: The arrangement of immunoglobulin and T cell receptor genes in human lymphoproliferative disorders. Adv Immunol 1987, 40:247-321 [DOI] [PubMed] [Google Scholar]
- 6.Medeiros LJ, Bagg A, Cossman J: Application of molecular genetics to the diagnosis of hematopoietic malignancies. Knowles DM eds. Neoplastic Hematopathology. 1992, :pp 263-298 Williams and Wilkins, Baltimore [Google Scholar]
- 7.Arber DA: Molecular diagnostic approach to non-Hodgkin’s lymphoma. J Mol Diagn 2000, 2:178-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sioutos N, Bagg A, Michaud GY, Irving SG, Hartmann DP, Siragy H, Oliveri DR, Locker J, Cossman J: Polymerase chain reaction versus Southern blot hybridization: detection of immunoglobulin heavy-chain gene rearrangements. Diagn Mol Pathol 1995, 4:8-13 [DOI] [PubMed] [Google Scholar]
- 9.Schroeder HW, Jr, Walter MA, Hofker MH, Ebens A, Willems van Dijk K, Liao LC, Cox DW, Milner EC, Perlmutter RM: Physical linkage of a human immunoglobulin heavy chain variable region gene segment to diversity and joining region elements. Proc Natl Acad Sci USA 1988, 85:8196-8200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler S, Jones CD, Warnke RA, Zehnder JL: PCR-heteroduplex analysis of T-cell receptor gamma gene rearrangement in paraffin-embedded skin biopsies. Am J Dermatopathol 2000, 22:321-327 [DOI] [PubMed] [Google Scholar]
- 11.Grosso LE, Collins BT: DNA polymerase chain reaction using fine needle aspiration biopsy smears to evaluate non-Hodgkin’s lymphoma. Acta Cytol 1999, 43:837-841 [DOI] [PubMed] [Google Scholar]
- 12.Ben-Ezra J: Variable rate of detection of immunoglobulin heavy chain V-D-J rearrangement by PCR: a systematic study of 41 B cell non-Hodgkin’s lymphomas and leukemias. Leuk Lymphoma 1992, 7:289-295 [DOI] [PubMed] [Google Scholar]
- 13.Diss TC, Peng H, Wotherspoon AC, Isaacson PG, Pan L: Detection of monoclonality in low-grade B cell lymphomas using the polymerase chain reaction is dependent on primer selection and lymphoma type. J Pathol 1993, 169:291-295 [DOI] [PubMed] [Google Scholar]
- 14.Arber DA, Braziel RA, Bagg A, Bijwaard KE: Evaluation of T cell receptor testing in lymphoid neoplasms: results of a multi-center study of 29 extracted DNA and paraffin-embedded samples. J Mol Diagn 2001, 3:133-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 16.Brisco MJ, Tan LW, Orsborn AM, Morley AA: Development of a highly sensitive assay, based on the polymerase chain reaction, for rare B lymphocyte clones in a polyclonal population. Br J Haematol 1990, 75:163-167 [DOI] [PubMed] [Google Scholar]
- 17.McCarthy KP, Sloane JP, Wiedemann LM: Rapid method for distinguishing clonal from polyclonal B cell populations in surgical biopsy specimens. J Clin Pathol 1990, 43:429-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achille A, Scarpa A, Montresor M, Scardoni M, Zamboni G, Chilosi M, Capelli P, Franzin G, Menestrina F: Routine application of polymerase chain reaction in the diagnosis of monoclonality of B cell lymphoid proliferations. Diagn Mol Pathol 1995, 4:14-24 [DOI] [PubMed] [Google Scholar]
- 19.Linke B, Pyttlich J, Tiemann M, Suttorp M, Parwaresch R, Hiddemann W, Kneba M: Identification and structural analysis of rearranged immunoglobulin heavy chain genes in lymphomas and leukemias. Leukemia 1995, 9:840-847 [PubMed] [Google Scholar]
- 20.Segal GH, Jorgensen T, Masih AS, Braylan RC: Optimal primer selection for clonality assessment by polymerase chain reaction analysis: I. low grade B cell lymphoproliferative disorders of nonfollicular center cell type. Hum Pathol 1994, 25:1269-1275 [DOI] [PubMed] [Google Scholar]
- 21.Reed TJ, Reid A, Wallberg K, O’Leary TJ, Frizzera G: Determination of B cell clonality in paraffin-embedded lymph nodes using the polymerase chain reaction. Diagn Mol Pathol 1993, 2:42-49 [PubMed] [Google Scholar]
- 22.Aubin J, Davi F, Nguyen-Salomon F, Leboeuf D, Debert C, Taher M, Valensi F, Canioni D, Brousse N, Varet B: Description of a novel FR1 IgH PCR strategy and its comparison with three other strategies for the detection of clonality in B cell malignancies. Leukemia 1995, 9:471-479 [PubMed] [Google Scholar]
- 23.NCCLS: Immunoglobulin and T Cell Receptor Gene Rearrangement Assays: Approved Guideline. NCCLS document MM2-A (ISBN 1–56238-289–6). Wayne, PA, NCCLS, 1995, 15:1–30
- 24.Deane M, McCarthy KP, Wiedemann LM, Norton JD: An improved method for detection of B-lymphoid clonality by polymerase chain reaction. Leukemia 1991, 5:726-730 [PubMed] [Google Scholar]
- 25.Wan JH, Trainor KJ, Brisco MJ, Morley AA: Monoclonality in B cell lymphoma detected in paraffin wax-embedded sections using the polymerase chain reaction J Clin Pathol 1990, 43:888-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inghirami G, Szabolcs MJ, Yee HT, Corradini P, Cesarman E, Knowles DM: Detection of immunoglobulin gene rearrangement of B cell non-Hodgkin’s lymphomas and leukemias in fresh, unfixed and formalin-fixed, paraffin-embedded tissue by polymerase chain reaction. Lab Invest 1993, 68:746-757 [PubMed] [Google Scholar]
- 27.Chen YT, Whitney KD, Chen Y: Clonality analysis of B cell lymphoma in fresh-frozen and paraffin-embedded tissues: the effects of variable polymerase chain reaction parameters. Mod Pathol 1994, 7:429-434 [PubMed] [Google Scholar]
- 28.Porter-Jordan K, Keiser JF, Garrett CT: Interfering substances that cause inhibition in polymerase chain reaction (PCR) assays. J Anal Chem 1990, 337:119-124 [Google Scholar]
- 29.Tbakhi A, Totos G, Pettay JD, Myles J, Tubbs RR: The effect of fixation on detection of B cell clonality by polymerase chain reaction. Mod Pathol 1999, 12:272-278 [PubMed] [Google Scholar]
- 30.Camilleri-Broet S, Devez F, Tissier F, Ducruit V, Le Tourneau A, Diebold J, Audouin J, Molina T: Quality control and sensitivity of polymerase chain reaction techniques for the assessment of immunoglobulin heavy chain gene rearrangements from fixed- and paraffin-embedded samples. Ann Diagn Pathol 2000, 4:71-76 [DOI] [PubMed] [Google Scholar]
- 31.Theriault C, Galoin S, Valmary S, Selves J, Lamant L, Roda D, Rigal-Huguet F, Brousset P, Delsol G, Al Saati T: PCR analysis of immunoglobulin heavy chain (IgH) and TCR-γ chain gene rearrangements in the diagnosis of lymphoproliferative disorders: results of a study of 525 cases. Mod Pathol 2000, 13:1269-1279 [DOI] [PubMed] [Google Scholar]
- 32.Derksen PW, Langerak AW, Kerkhof E, Wolvers-Tettero IL, Boor PP, Mulder AH, Vrints LW, Coebergh JW, van Krieken JH, Schuuring E, Kluin PM, van Dongen JJ: Comparison of different polymerase chain reaction-based approaches for clonality assessment of immunoglobulin heavy-chain gene rearrangements in B cell neoplasia. Mod Pathol 1999, 12:794-805 [PubMed] [Google Scholar]
- 33.Kuppers R, Zhao M, Hansmann ML, Rajewsky K: Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J 1993, 12:4955-4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lombardo JF, Hwang TS, Maiese RL, Millson A, Segal GH: Optimal primer selection for clonality assessment by polymerase chain reaction analysis: III. intermediate and high-grade B cell neoplasms. Hum Pathol 1996, 27:373-380 [DOI] [PubMed] [Google Scholar]
- 35.Kuppers R, Zhao M, Rajewsky K, Hansmann ML: Detection of clonal B cell populations in paraffin-embedded tissues by polymerase chain reaction. Am J Pathol 1993, 143:230-239 [PMC free article] [PubMed] [Google Scholar]
- 36.Gleissner B, Maurer J, Thiel E: Detection of immunoglobulin heavy chain genes rearrangements in B cell leukemias, lymphomas, multiple myelomas, monoclonal, and polyclonal gammopathies. Leuk Lymphoma 2000, 39:151-155 [DOI] [PubMed] [Google Scholar]
- 37.Gong JZ, Zheng S, Chiarle R, De Wolf-Peeters C, Palestro G, Frizzera G, Inghirami G: Detection of immunoglobulin kappa light chain rearrangements by polymerase chain reaction: an improved method for detecting clonal B cell lymphoproliferative disorders. Am J Pathol 1999, 155:355-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramasamy I, Brisco M, Morley A: Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol 1992, 45:770-775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelicci P-G, Knowles DMII, Dalla Favera R: Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med 1985, 162:1015-1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor JM, Spagnolo DV, Kay PH: B cell target DNA quantity is a critical factor in the interpretation of B cell clonality by PCR. Pathology 1997, 29:309-312 [DOI] [PubMed] [Google Scholar]
- 41.Elenitoba-Johnson KS, Bohling SD, Mitchell RS, Brown MS, Robetorye RS: PCR analysis of the immunoglobulin heavy chain gene in polyclonal processes can yield pseudoclonal bands as an artifact of low B cell number. J Mol Diagn 2000, 2:92-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoeve MA, Krol AD, Philippo K, Derksen PW, Veenendaal RA, Schuuring E, Kluin PM, van Krieken JH: Limitations of clonality analysis of B cell proliferations using CDR3 polymerase chain reaction. Mol Pathol 2000, 53:194-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson PW, Swinbank K, MacLennan S, Colomer D, Debuire B, Diss T, Gabert J, Gupta RK, Haynes A, Kneba M, Lee MS, Macintyre E, Mensink E, Moos M, Morgan GJ, Neri A, Johnson A, Reato G, Salles G, van’t Veer MB, Zehnder JL, Zucca E, Selby PJ, Cotter FE: Variability of polymerase chain reaction detection of the bcl-2-IgH translocation in an international multi-centre study. Ann Oncol 199, 10:1349–1354 [DOI] [PubMed]
- 44.Cabras AD, Kremer M, Schulz S, Werner M, Hummel M, Komminoth P, Hofler G, Hofler H: Quality assessment in diagnostic molecular pathology: experience from a German-Austrian-Swiss multi-center trial. Virchows Arch 2000, 437:46-51 [DOI] [PubMed] [Google Scholar]
- 45.Burmeister T, Maurer J, Aivado M, Elmaagacli AH, Grunebach F, Held KR, Hess G, Hochhaus A, Hoppner W, Lentes KU, Lubbert M, Schafer KL, Schafhausen P, Schmidt CA, Schuler F, Seeger K, Seelig R, Thiede C, Viehmann S, Weber C, Wilhelm S, Christmann A, Clement JH, Ebener U, Enczmann J, Leo R, Schleuning M, Schoch R, Thiel E: Quality assurance in RT-PCR-based BCR/ABL diagnostics: results of an inter-laboratory test and a standardization approach. Leukemia. 2000, 14:1850-1856 [DOI] [PubMed] [Google Scholar]
- 46.Bolufer P, Lo Coco F, Grimwade D, Barragan E, Diverio D, Cassinat B, Chomienne C, Gonzalez M, Colomer D, Gomez MT, Marugan I, Roman J, Delgado MD, Garcia-Marco JA, Bornstein R, Vizmanos JL, Martinez B, Jansen J, Villegas A, de Blas JM, Cabello P, Sanz MA: Variability in the levels of PML-RARa fusion transcripts detected by the laboratories participating in an external quality control program using several reverse transcription polymerase chain reaction protocols. Haematologica 2001, 86:570-576 [PubMed] [Google Scholar]
- 47.van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G, Griesinger F, Parreira A, Gameiro P, Diaz MG, Malec M, Langerak AW, San Miguel JF, Biondi A: Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia 1999, 13:1901-1928 [DOI] [PubMed] [Google Scholar]