Abstract
Acinetobacter sp. strain DSM 17874 is capable of utilizing n-alkanes with chain lengths ranging from that of decane (C10H22) to that of tetracontane (C40H82) as a sole carbon source. Two genes encoding AlkB-type alkane hydroxylase homologues, designated alkMa and alkMb, have been shown to be involved in the degradation of n-alkanes with chain lengths of from 10 to 20 C atoms in this strain. Here, we describe a novel high-throughput screening method and the screening of a transposon mutant library to identify genes involved in the degradation of n-alkanes with C chain lengths longer than 20, which are solid at 30°C, the optimal growth temperature for Acinetobacter sp. strain DSM 17874. A library consisting of approximately 6,800 Acinetobacter sp. strain DSM 17874 transposon mutants was constructed and screened for mutants unable to grow on dotriacontane (C32H66) while simultaneously showing wild-type growth characteristics on shorter-chain n-alkanes. For 23 such mutants isolated, the genes inactivated by transposon insertion were identified. Targeted inactivation and complementation studies of one of these genes, designated almA and encoding a putative flavin-binding monooxygenase, confirmed its involvement in the strain's metabolism of long-chain n-alkanes. To our knowledge, almA represents the first cloned gene shown to be involved in the bacterial degradation of long-chain n-alkanes of 32 C's and longer. Genes encoding AlmA homologues were also identified in other long-chain n-alkane-degrading Acinetobacter strains.
Long-chain (LC) alkanes, with chain lengths of >20 C atoms, are environmental pollutants and may also cause problems in recovery, transportation, and processing of crude oil by e.g., clogging pipes. The possibilities of using processes based on the microbial biodegradation of hydrocarbons for removal of pollutants from the environment and upgrading oil refinery products have been suggested (27). Several bacterial enzymes for aerobic degradation of alkanes have been identified, e.g., cytochrome P450 (11), monooxygenase (6), and dioxygenase (10). The best-characterized system for alkane degradation is the Alk system of Pseudomonas putida GPo1 (26), sequentially converting alkanes to the corresponding alcohols, aldehydes, carboxylic acids, and acyl-coenzyme A's (CoAs), which then enter the β-oxidation pathway. Most of these systems catalyze the degradation of relatively short-chain alkanes, and very little is known about enzymes involved in the degradation of LC alkanes.
Strains of the genus Acinetobacter, capable of utilizing alkanes with C chain lengths ranging from 10 to 44 have been described (1, 12, 18, 21, 24). Acinetobacter sp. strain DSM 17874 (initially described as A. venetianus 6A2 [24]) is capable of utilizing C10 to C40 n-alkanes as a sole carbon source. We have recently identified two alkB paralogs, alkMa and alkMb, which were shown to be involved in the utilization of n-alkanes with C chain lengths up to 20 in this strain (24). Furthermore, we postulated the existence of at least one other enzyme system in Acinetobacter sp. strain DSM 17874 involved in degradation of LC alkanes.
Here we describe the construction and high-throughput screening (HTS) of a library of transposon mutants of Acinetobacter sp. strain DSM 17874 (24), which have led to identification of several genes possibly involved in LC alkane degradation. Detailed analysis of one of these genes, designated almA, confirmed its involvement in the degradation of LC alkanes with C chain lengths of 32 and longer. Interestingly, the almA-deficient mutant, MAV1, could still grow with C24 and shorter alkanes as a sole carbon source, indicating the occurrence of yet another enzyme system for the degradation of C20 and longer alkanes in Acinetobacter sp. strain DSM 17874.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains, plasmids, and oligonucleotide primers used in this study are presented in Table 1.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotide primers used in this study
| Strain, plasmid, or primer | Source and/or reference; description or sequence (5′-); restriction sitea |
|---|---|
| Bacterial strains | |
| Acinetobacter sp. strain DSM 17874 | 24; Camr, deposited in the DSMZ strain collection under accession no. DSM17874 |
| Escherichia coli S17-1 (λpir) | 4 |
| Acinetobacter baylyi ADP1 | A. Steinbüchel, 8 |
| Acinetobacter sp. strain M-1 | Y. Sakai, 21 |
| Acinetobacter sp. strain RAG-1 | Purchased from ATCC, accession no. ATCC31012 |
| Acinetobacter sp. strain MAV1 | This study; almA knockout mutant, Camr Aprr |
| Acinetobacter sp. strain BKO2 | This study; almB knockout mutant, Camr Aprr |
| Plasmids | |
| pLOFKm | 7; mini-Tn10 delivery vector, Kanr |
| pSOK201 | 28; Aprr |
| pSOK804 | 3; Aprr |
| pDLM02.1 | 13; A. baylyi ADP1 and ColE1 replication origin, Tdk/Kanr selection/counter-selection cassette, Ampr |
| pLAL50 | This study; part of pSOK804 containing a 607-bp almA fragment, Aprr |
| pBKO2 | This study; derivative of pSOK201 containing a 300-bp orf1 fragment, Aprr |
| pALMA1 | This study; derivative of pDLM02.1 carrying the almA gene from Acinetobacter sp. strain 6A2, Ampr Kanr |
| pACIAD1 | This study; derivative of pDLM02.1 carrying the ACIAD3192 gene from A. baylyi ADP1, Ampr Kanr |
| Primers | |
| Tn10:1 | GGATCATATGACAAGATGTG |
| Kan1 | GCTCTAGACCGTCAAGTCAGCGTAATGC |
| almA1 | GACATGTGTATTGTCAAATTTGTGC |
| almA2 | CCAATGAGATCATGGAAGAAC |
| almA3 | GCTCTAGACTATCCTGGTATTCGTTCAG; XbaI |
| almA4 | CGGGATCCTAAATACCACGTTGCATACC; BamHI |
| almA5 | GCGCGCGCATGCGACATGTGTATTGTCAAATTTGTGC; SphI |
| almA6 | GCGCGCGGCGCCCCCTATGCCATGCATAGGGTTTC; NarI |
| orf1:1 | GCGCGCGCATGCGACAGCAATTGCTGAGAAGTTGG; SphI |
| orf1:2 | GCGCGCGAATTCGTGAAATGAGCAACCTCTCCTCC; EcoRI |
| ACIAD1 | GCGCGCGCATGCCGCGAAGTAAACACTTCAGCAG; SphI |
| ACIAD2 | GCGCGCGGCGCCCCTTTTTTTGTTTAGCTCAAGTTAGGATAC; NarI |
Antibiotic resistance markers: Ampr, ampicillin resistance; Camr, chloramphenicol resistance; Kanr, kanamycin resistance; Aprr, apramycin resistance. Restriction sites are underlined.
Media and growth conditions.
Bacterial strains were grown in Luria broth (LB; 10 g tryptone [Oxoid], 5 g yeast extract [Oxoid], and 5 g NaCl per liter of deionized water) or Czapek broth (CB; 3 g NaNO3, 1 g K2PO4, 0.5 g MgSO4, 0.5 g KCl, and 0.01 g FeSO4 per liter of deionized water, pH 7.5, supplemented with various carbon sources). The same media were solidified with 15 g agar per liter medium to make plates. Antibiotics at the following concentrations were used when appropriate: ampicillin, 100 mg/liter; apramycin, 50 mg/liter; chloramphenicol, 30 mg/liter; and kanamycin, 25 mg/liter. n-Alkanes of defined chain lengths were purchased from Sigma-Aldrich. They will be referred to by the number of carbon atoms they contain, e.g., decane will be referred to as C10, throughout the paper. Growth of Acinetobacter strains in liquid and on solid media supplemented with n-alkanes was carried out as described before (24).
Construction of an Acinetobacter sp. strain DSM 17874 transposon mutant library.
Plasmid pLOFKm (7), carrying a mini-Tn10 delivery system, was used to construct an Acinetobacter sp. strain DSM 17874 transposon mutant library. The pLOFKm plasmid contains a mini-Tn10 transposon harboring a kanamycin resistance marker, the conjugal transfer origin oriT, and the Tn10 transposase under the control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter. This plasmid cannot replicate in Acinetobacter sp. strain DSM 17874. pLOFKm was introduced into Acinetobacter sp. strain DSM 17874 via conjugation. Escherichia coli S17-1 (λpir)/pLOFKm and Acinetobacter sp. strain DSM 17874 were grown in 50 ml LB containing the appropriate antibiotics in 500-ml baffled Erlenmeyer flasks at 30°C on a rotary shaker at 200 rpm. When the cultures had reached an optical density at 600 nm of about 0.4, several aliquots of 1 ml of each culture were mixed and immediately centrifuged at 8,000 rpm for 5 min. The pellets were resuspended in 100 μl LB and placed as drops on LB plates supplemented with 500 μM IPTG to induce the expression of the transposase. The plates were incubated at 30°C overnight. Bacteria were harvested from the plates and resuspended in LB medium. The resulting cell suspension was diluted and spread onto LB plates supplemented with kanamycin and chloramphenicol to select against the E. coli donor cells (24). The plates were incubated at 30°C for about 48 h, and colonies were picked using a QPix robot (Genetix) and transferred into 96-well microtiter plates (Nunc) containing 120 μl LB supplemented with chloramphenicol and kanamycin in each well. All liquid handling using 96-well microtiter plates was carried out using a Genesis RSP200 robot (Tecan). The plates were incubated at 30°C and 900 rpm in a Multitron shaking incubator (Infors) for 24 h. A library containing approximately 6,800 Acinetobacter sp. strain DSM 17874 transposon mutants was created.
HTS of the Acinetobacter sp. strain DSM 17874 transposon mutant library for mutants deficient in LC alkane degradation.
Transposon mutants from the 96-well library plates were replicated onto Omnitray plates (Nunc) containing solid CB medium without a carbon source. After transfer of the mutants, C32 alkane was added as a powder to the Omnitray plates as described before (24). The plates were incubated at 30°C for 48 h, and growth was detected by overlaying the plates with a top agar containing 0.05% (wt/vol) iodonitrotetrazolium chloride (INT). Reduction of INT by the active respiratory chain of growing cells led to purple staining of colonies (5). Mutants not showing growth on the C32 alkane were rearrayed using the QPix robot from the original library into new 96-well microtiter plates containing 120 μl LB, supplemented with chloramphenicol and kanamycin in each well. The plates were incubated overnight at 30°C and 900 rpm, resulting in a library enriched for mutants no longer capable of utilizing C32 as a carbon source. To confirm the initial screening results and further analyze these mutants, they were replicated onto Omnitray plates containing CB supplemented with 0.5% (wt/vol) sodium acetate, C16, or C32 alkanes as a sole carbon source. Mutants showing no growth on C32 and coincidentally growing at wild-type level on plates supplemented with acetate or C16 were chosen for further analysis.
Molecular biology methods.
Total chromosomal DNA was isolated from Acinetobacter strains using the QIAGEN DNeasy tissue kit. PCR amplification and sequencing of chromosomal regions flanking the transposons of selected Acinetobacter sp. strain DSM 17874 mutants were carried out by inverse PCR (15) followed by ABI sequencing using primers Tn10:1 and Kan1 (Table 1), specific for the transposon sequence. PCR and subsequent sequencing of the almA region from Acinetobacter sp. strain DSM 17874 and homologous regions from Acinetobacter sp. strain M-1 and Acinetobacter sp. strain RAG-1 were carried out using primers almA1 and almA2 (Table 1), followed by primer walking. The sequence of the Acinetobacter baylyi ADP1 ACIAD3192 gene was obtained from GenBank, accession no. NC_005966 (2).
Construction of the Acinetobacter sp. strain DSM 17874 almA and orf1 disruption mutants, MAV1 and BKO2.
Suicide vectors pLAL50 and pBKO2, for disruption of almA and orf1, respectively, in Acinetobacter sp. strain DSM 17874 were constructed. A 607-bp XbaI-BamHI almA fragment and a 300-bp SphI-EcoRI orf1 fragment were PCR amplified using Acinetobacter sp. strain DSM 17874 chromosomal DNA as the template and the primer pairs almA3 and almA4 for almA amplification and orf1:1 and orf1:2 for orf1 amplification (Table 1). The almA PCR fragment was digested with XbaI and BamHI and ligated into the 3-kb XbaI-BamHI fragment of pSOK804 (which is identical to that of pSOK201 [28]) containing the ColE1 ori, oriT, and an apramycin resistance gene, resulting in plasmid pLAL50. The orf1 PCR product was digested with SphI and EcoRI and cloned into the 3-kb SphI-EcoRI fragment of pSOK201 (28) to give plasmid pBKO2. Each plasmid was first transferred into E. coli S17-1 (λpir) via heat shock transformation and subsequently transferred into Acinetobacter sp. strain DSM 17874 by conjugation. Transconjugants were selected on LB plates containing apramycin and chloramphenicol. Integration of the plasmids into the Acinetobacter sp. strain DSM 17874 chromosome resulted in the almA and orf1 disruption mutants, MAV1 and BKO2, respectively. The insertion of the respective vectors into the Acinetobacter sp. strain DSM 17874 chromosome was confirmed by Southern blot analysis using the DIG nonradioactive nucleic acid labeling and detection system (Roche) and probes for detection of almA or orf1.
Construction of plasmids pALMA1 and pACIAD1 for complementation studies.
The almA gene of Acinetobacter sp. strain DSM 17874 was amplified by PCR using the primers almA5 and almA6 (Table 1) and chromosomal DNA from Acinetobacter sp. strain DSM 17874 as a template. The PCR product was digested with SphI and NarI and ligated into similarly digested vector pDLM02.1, giving plasmid pALMA1.
The ACIAD3192 gene from A. baylyi ADP1 was amplified by PCR using the primers ACIAD1 and ACIAD2 (Table 1) and chromosomal DNA from A. baylyi ADP1 as a template. The PCR product was digested with SphI and NarI and ligated into similarly digested vector pDLM02.1, giving plasmid pACIAD1.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences reported in this paper are EF212873 for the Acinetobacter sp. strain DSM 17874 almA region, EF212874 for the Acinetobacter sp. strain RAG-1 almA region, and EF212875 for the Acinetobacter sp. strain M-1 almA region.
RESULTS
A novel high-throughput screening method allows identification of genes involved in n-alkane degradation in Acinetobacter sp. strain DSM 17874.
The presence of at least one metabolic pathway for degradation of n-alkanes with chain lengths of over 20 C atoms in Acinetobacter sp. strain DSM 17874 has been postulated (24). To identify genes involved in the metabolism of LC alkanes in Acinetobacter sp. strain DSM 17874, we have designed and used a novel HTS method for screening an Acinetobacter sp. strain DSM 17874 transposon mutant library for mutants which can no longer utilize solid LC alkanes as a sole carbon source. This screening yielded 34 mutants showing no significant growth with C32 as a sole carbon source but wild-type-like growth with acetate or C16 as a sole carbon source. The mutants were analyzed for the site of transposon insertion, and 16 different putative genes were identified (Table 2). All of these genes showed highest homology to genes from other Acinetobacter strains, predominantly A. baylyi ADP1, for which the entire genome has been sequenced (2). One mutant harbored the transposon insertion within a gene encoding a homologue of a putative flavin-binding monooxygenase, ACIAD3192 from A. baylyi ADP1. This gene, designated almA (n-alkane metabolism A), was chosen for further analysis.
TABLE 2.
Genes identified in HTS of the Acinetobacter sp. strain DSM 17874 transposon mutant library
| Genea | Organism | Function or functional category | No. of mutantsb | % Identity/ length (bp) |
|---|---|---|---|---|
| ACIAD3192 | A. baylyi ADP1 | Putative monooxygenase | 1 | 81-91/35-447 |
| gacS/barA | A. baylyi ADP1 | GacS-like sensor kinase protein | 5 | 79-96/71-157 |
| xcpS | A. baylyi ADP1 | General secretion pathway protein F | 1 | 82-84/107-152 |
| xcpR | A. baylyi ADP1 | General secretion pathway protein E, putative ATPase | 2 | 87-88/61-115 |
| ACIAD0294 | A. baylyi ADP1 | General secretion pathway protein | 2 | 80/121-209 |
| 32_384 | A. baumannii AYE | Putative major facilitator superfamily drug transporter | 1 | 80/257 |
| filA | Acinetobacter sp. strain BD413 | Pilus assembly system fil gene cluster | 1 | 96/29 |
| ACIAD0505 | A. baylyi ADP1 | Formyltetrahydrofolate deformylase | 1 | 86/365 |
| ACIAD2911, panD | A. baylyi ADP1 | Aspartate 1-decarboxylase precursor CoA | 1 | 83/179 |
| ugd | Acinetobacter sp. strain RAG-1 | UDP-glucose DH (close to the wee gene cluster for emulsan production) | 1 | 100/461 |
| himD | A. baylyi ADP1 | Integration host factor, β-SU | 1 | 83/218 |
| ACIAD0049 | A. baylyi ADP1 | Putative lineoyl-CoA desaturase | 1 | 80-91/43-187 |
| ACIAD0473 | A. baylyi ADP1 | Putative trancriptional regulator (AraC family) | 2 | 83-96/26-136 |
| truA | A. baylyi ADP1 | tRNA-pseudouridine synthase | 1 | 84/150 |
| acnA | A. baylyi ADP1 | Aconitate hydratase | 1 | 81-84/242-294 |
| ACIAD3547 | A. baylyi ADP1 | Hypothetical protein, putative enzyme | 1 | 84/140 |
Gene with closest homology in BLASTn homology search.
For six mutants, there was no reliable match; five mutants could not be analyzed.
The almA locus is present in other Acinetobacter spp. capable of degrading LC alkanes.
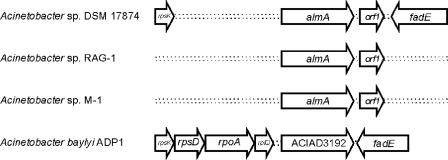
The almA gene and a part of the surrounding chromosomal region in Acinetobacter sp. strain DSM 17874 were sequenced, and the sequence of the surrounding region was found to be very similar to that of the region surrounding A. baylyi ADP1 ACIAD3192 (Fig. 1), with the exception of the presence of an additional open reading frame 127 bp downstream of almA in Acinetobacter sp. strain DSM 17874. The latter putative gene, designated orf1, presumably encodes a 166-amino-acid polypeptide, but no significant homology to any known gene or protein could be found in the GenBank database.
FIG. 1.
Schematic representation of the almA regions in different Acinetobacter strains. Arrows indicate the relative orientations of the genes. Gaps indicate estimated distances. The Acinetobacter sp. strain DSM 17874 almA coding region is 1,491 bp and shows 74.8% sequence identity to ACIAD3192 from A. baylyi ADP1. The identity between the deduced AlmA and ACIAD3192 peptide sequences is 76.7%. The almA and orf1 genes from Acinetobacter sp. strain RAG-1 showed 100% nucleotide sequence identity to Acinetobacter sp. strain DSM 17874. The nucleotide sequence identity between Acinetobacter sp. strain DSM 17874 and Acinetobacter sp. strain M-1 was found to be 85.7% for almA and 75.5% for orf1, with predicted peptide sequence identities of 95.4% for AlmA and 80.1% for Orf1.
A. baylyi ADP1 and Acinetobacter sp. strains RAG-1 and M-1 were also found to grow with C32 and C36, respectively, as a sole carbon source (our unpublished data; 21). Genes homologous to almA and orf1 were also identified in Acinetobacter sp. strain RAG-1 and Acinetobacter sp. strain M-1 (Fig. 1). Several AlmA homologues identified in the GenBank database were phylogenetically analyzed using MEGA version 3.1 (9) and the neighbor-joining algorithm (20). The AlmA homologues from Acinetobacter sp. strain DSM 17874, A. baylyi ADP1, and Acinetobacter sp. strains RAG-1 and M-1 clustered together with homologues from, e.g., Marinobacter aquaeolei VT8, Alcanivorax borkumensis SK2 (two proteins), Oceanobacter sp. strain RED65, Ralstonia spp., Mycobacterium spp., a Photorhabdus sp., Psychrobacter spp., and Nocardia farcinica IFM10152 as close neighbors (data not shown). All of these are annotated solely according to sequence homology, and functions have not yet been experimentally confirmed.
AlmA is involved in the degradation of LC alkanes in Acinetobacter sp. strain DSM 17874.
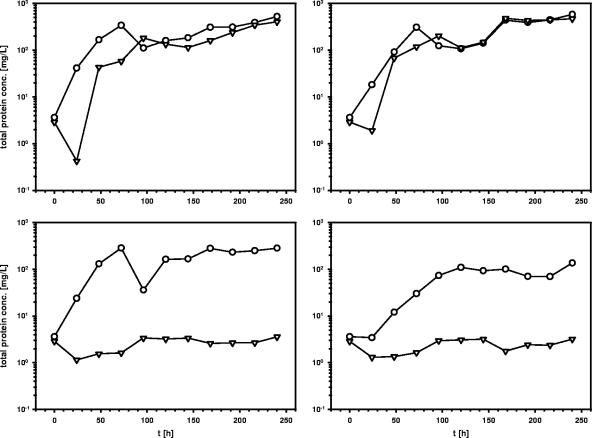
To analyze the function of the almA gene in LC alkane utilization, the Acinetobacter sp. strain DSM 17874 almA mutant MAV1 was constructed and used in growth experiments with various n-alkanes as a sole carbon source. MAV1 did not grow with C32 and C36 alkanes as a sole carbon source, while showing wild-type growth with C20 and C24 alkanes (Fig. 2), indicating that AlmA functions specifically in LC alkane utilization in Acinetobacter sp. strain DSM 17874.
FIG. 2.
Growth of Acinetobacter sp. strain DSM 17874 (wild-type; ○) and Acinetobacter sp. strain MAV1 (almA-deficient mutant; ▿) in 100 ml CB medium supplemented with seven 50-μl solid droplets of C20 (top left), C24 (top right), C32 (bottom left), or C36 (bottom right). Growth was measured as an increase in protein in the cultures over time. The values presented are the averages of two independent experiments.
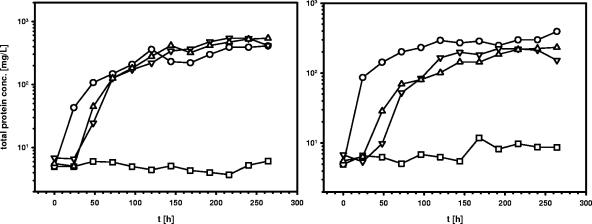
Introduction of the almA gene on a plasmid in the almA-deficient strain MAV1 restored the strain's ability to grow with C32 and C36 alkanes as a sole carbon source to almost wild-type level (Fig. 3). This result confirmed that the LC alkane degradation deficiency of MAV1 is not caused by a polar effect on the genes downstream of almA and that the almA deficiency is responsible for the inability of the MAV1 mutant to utilize LC alkanes.
FIG. 3.
Complementation of almA activity in the almA-deficient mutant Acinetobacter sp. strain MAV1 with the almA gene on a plasmid (see Table 1). Shown are data on the growth of Acinetobacter sp. strain DSM 17874/pDLM02.1 (○), Acinetobacter sp. strain MAV1/pDLM02.1 (□), Acinetobacter sp. strain MAV1/pALMA1 (▿), and Acinetobacter sp. strain MAV1/pACIAD1 (▵) in 100 ml CB medium supplemented with seven 50-μl solid droplets of C32 (left) or C36 (right). Growth was measured as an increase in protein in the cultures over time. The values presented are the averages of two independent experiments.
The ACIAD3192 gene from A. baylyi ADP1 represents the closest homologue to the almA gene found in the databases. Therefore, this gene was chosen for heterologous functional complementation of the almA-deficient mutant MAV1. Acinetobacter sp. strain MAV1 carrying the ACIAD3192 gene on a plasmid showed growth with C32 and C36 alkanes (Fig. 3), clearly showing that the product of this gene is a functional homologue of AlmA in the LC alkane utilization pathway.
To investigate whether orf1 is involved in n-alkane metabolism by Acinetobacter sp. strain DSM 17874, an orf1 disruption mutant strain, BKO2, was constructed. Growth of BKO2 was found to be indistinguishable from that of the wild-type strain with C20, C24, C32, and C36 alkanes as sole carbon sources (data not shown), suggesting that orf1 most likely is not involved in the strain's alkane metabolism, at least not under the conditions tested.
DISCUSSION
We here describe the identification of novel genes involved in LC alkane degradation by Acinetobacter sp. strain DSM 17874 using HTS of a transposon mutant library consisting of ca. 6,800 mutants. Although this number of mutants most likely does not cover insertions into all of the strain's nonessential genes, it allowed identification of several genes presumably involved in LC alkane degradation (Table 2). Some of the genes identified here have been found to be involved in (short-chain) alkane utilization previously, e.g., the general secretion pathway gene xcpR (16), the regulator of the AraC family (17), and the ugd gene (14). Further investigation is necessary to confirm and analyze the involvement of these genes specifically in alkane metabolism in strain DSM 17874. We analyzed the involvement of the newly identified almA gene in LC alkane degradation in more detail. This gene is homologous to the ACIAD3192 gene from A. baylyi ADP1 and encodes a putative flavin-binding monooxygenase. The majority of the enzymes reported to be involved in the initial step of aerobic alkane metabolism are represented by monooxygenases/hydroxylases (6, 17, 23, 25), and for Alcanivorax borkumensis SK2, a number of putative monooxygenases and oxidoreductases have been implicated in alkane degradation (19, 22).
The involvement of AlmA in the utilization of C32 and C36 alkanes by Acinetobacter sp. strain DSM 17874 was confirmed by mutational analysis and complementation studies. Interestingly, the almA-deficient mutant could still grow on alkanes with a C chain length up to 24, indicating the presence of at least one more enzyme system involved in the degradation of C20 to C24 alkanes in the strain.
The LC alkane utilization deficiency of the almA disruption mutant could also be complemented by the ACIAD3192 gene from A. baylyi ADP1, suggesting a similar function for the latter gene in ADP1. The present study demonstrates the utility of the novel HTS system and paves the way for comprehensive analysis of genes and enzymes involved in bacterial alkane utilization.
Acknowledgments
This project was supported by the VISTA foundation and Statoil ASA.
We thank A. Steinbüchel (University of Münster, Germany) for providing A. baylyi ADP1, Y. Sakai (Graduate School of Agriculture, Kyoto University, Japan) for providing Acinetobacter sp. strain M-1, J. M. Bacher (The Scripps Research Institute, La Jolla, CA) for providing plasmid pDLM02.1 (13), and A. Winnberg and G. Klinkenberg (SINTEF Materials and Chemistry) for their assistance during the high-throughput screening experiments.
Footnotes
Published ahead of print on 30 March 2007.
REFERENCES
- 1.Asperger, O., A. Naumann, and H. P. Kleber. 1981. Occurrence of cytochrome P-450 in Acinetobacter strains after growth on N-hexadecane. FEMS Microbiol. Lett. 11:309-312. [Google Scholar]
- 2.Barbe, V., D. Vallenet, N. Fonknechten, A. Kreimeyer, S. Oztas, L. Labarre, S. Cruveiller, C. Robert, S. Duprat, P. Wincker, L. N. Ornston, J. Weissenbach, P. Marliere, G. N. Cohen, and C. Medigue. 2004. Unique features revealed by the genome sequence of Acinetobacter sp. ADP1, a versatile and naturally transformation competent bacterium. Nucleic Acids Res. 32:5766-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brautaset, T., S. E. Borgos, H. Sletta, T. E. Ellingsen, and S. B. Zotchev. 2003. Site-specific mutagenesis and domain substitutions in the loading module of the nystatin polyketide synthase, and their effects on nystatin biosynthesis in Streptomyces noursei. J. Biol. Chem. 278:14913-14919. [DOI] [PubMed] [Google Scholar]
- 4.de Lorenzo, V., L. Eltis, B. Kessler, and K. N. Timmis. 1993. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene 123:17-24. [DOI] [PubMed] [Google Scholar]
- 5.Haines, J. R., B. A. Wrenn, E. L. Holder, K. L. Strohmeier, R. T. Herrington, and A. D. Venosa. 1996. Measurement of hydrocarbon-degrading microbial populations by a 96-well plate most-probable-number procedure. J. Ind. Microbiol. 16:36-41. [DOI] [PubMed] [Google Scholar]
- 6.Hamamura, N., C. M. Yeager, and D. J. Arp. 2001. Two distinct monooxygenases for alkane oxidation in Nocardioides sp. strain CF8. Appl. Environ. Microbiol. 67:4992-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juni, E., and A. Janik. 1969. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum). J. Bacteriol. 98:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 10.Maeng, J. H., Y. Sakai, Y. Tani, and N. Kato. 1996. Isolation and characterization of a novel oxygenase that catalyzes the first step of n-alkane oxidation in Acinetobacter sp. strain M-1. J. Bacteriol. 178:3695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier, T., H. H. Forster, O. Asperger, and U. Hahn. 2001. Molecular characterization of the 56-kDa CYP153 from Acinetobacter sp. EB104. Biochem. Biophys. Res. Commun. 286:652-658. [DOI] [PubMed] [Google Scholar]
- 12.Makula, R. A., P. J. Lockwood, and W. R. Finnerty. 1975. Comparative analysis of the lipids of Acinetobacter species grown on hexadecane. J. Bacteriol. 121:250-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzgar, D., J. M. Bacher, V. Pezo, J. Reader, V. Doring, P. Schimmel, P. Marliere, and V. de Crecy-Lagard. 2004. Acinetobacter sp. ADP1: an ideal model organism for genetic analysis and genome engineering. Nucleic Acids Res. 32:5780-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakar, D., and D. L. Gutnick. 2001. Analysis of the wee gene cluster responsible for the biosynthesis of the polymeric bioemulsifier from the oil-degrading strain Acinetobacter lwoffii RAG-1. Microbiology 147:1937-1946. [DOI] [PubMed] [Google Scholar]
- 15.Ochman, H., J. W. Ajioka, D. Garza, and D. L. Hartl. 1990. Inverse polymerase chain reaction. Bio/Technology 8:759-760. [DOI] [PubMed] [Google Scholar]
- 16.Parche, S., W. Geissdorfer, and W. Hillen. 1997. Identification and characterization of xcpR encoding a subunit of the general secretory pathway necessary for dodecane degradation in Acinetobacter calcoaceticus ADP1. J. Bacteriol. 179:4631-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratajczak, A., W. Geissdorfer, and W. Hillen. 1998. Expression of alkane hydroxylase from Acinetobacter sp. strain ADP1 is induced by a broad range of n-alkanes and requires the transcriptional activator AlkR. J. Bacteriol. 180:5822-5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg, M., D. Gutnick, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 19.Sabirova, J. S., M. Ferrer, D. Regenhardt, K. N. Timmis, and P. N. Golyshin. 2006. Proteomic insights into metabolic adaptations in Alcanivorax borkumensis induced by alkane utilization. J. Bacteriol. 188:3763-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 21.Sakai, Y., J. H. Maeng, Y. Tani, and N. Kato. 1994. Use of long-chain n-alkanes (C13-C44) by an isolate, Acinetobacter sp. M-1. Biosci. Biotechnol. Biochem. 58:2128-2130. [Google Scholar]
- 22.Schneiker, S., V. A. Martins dos Santos, D. Bartels, T. Bekel, M. Brecht, J. Buhrmester, T. N. Chernikova, R. Denaro, M. Ferrer, C. Gertler, A. Goesmann, O. V. Golyshina, F. Kaminski, A. N. Khachane, S. Lang, B. Linke, A. C. McHardy, F. Meyer, T. Nechitaylo, A. Puhler, D. Regenhardt, O. Rupp, J. S. Sabirova, W. Selbitschka, M. M. Yakimov, K. N. Timmis, F. J. Vorholter, S. Weidner, O. Kaiser, and P. N. Golyshin. 2006. Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat. Biotechnol. 24:997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits, T. H., S. B. Balada, B. Witholt, and J. B. van Beilen. 2002. Functional analysis of alkane hydroxylases from gram-negative and gram-positive bacteria. J. Bacteriol. 184:1733-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Throne-Holst, M., S. Markussen, A. Winnberg, T. E. Ellingsen, H. K. Kotlar, and S. B. Zotchev. 2006. Utilization of n-alkanes by a newly isolated strain of Acinetobacter venetianus: the role of two AlkB-type alkane hydroxylases. Appl. Microbiol. Biotechnol. 72:353-360. [DOI] [PubMed] [Google Scholar]
- 25.van Beilen, J. B., E. G. Funhoff, A. van Loon, A. Just, L. Kaysser, M. Bouza, R. Holtackers, M. Rothlisberger, Z. Li, and B. Witholt. 2006. Cytochrome P450 alkane hydroxylases of the CYP153 family are common in alkane-degrading eubacteria lacking integral membrane alkane hydroxylases. Appl. Environ. Microbiol. 72:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Beilen, J. B., M. G. Wubbolts, and B. Witholt. 1994. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation 5:161-174. [DOI] [PubMed] [Google Scholar]
- 27.Van Hamme, J. D., A. Singh, and O. P. Ward. 2003. Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 67:503-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zotchev, S., K. Haugan, O. Sekurova, H. Sletta, T. E. Ellingsen, and S. Valla. 2000. Identification of a gene cluster for antibacterial polyketide-derived antibiotic biosynthesis in the nystatin producer Streptomyces noursei ATCC 11455. Microbiology 146(Pt. 3):611-619. [DOI] [PubMed] [Google Scholar]