Abstract
N-Octanoyl cyclopentylamide (C8-CPA) was found to moderately inhibit quorum sensing in Pseudomonas aeruginosa PAO1. To obtain more powerful inhibitors, a series of structural analogs of C8-CPA were synthesized and examined for their ability to inhibit quorum sensing in P. aeruginosa PAO1. The lasB-lacZ and rhlA-lacZ reporter assays revealed that the chain length and the ring structure were critical for C8-CPA analogs to inhibit quorum sensing. N-Decanoyl cyclopentylamide (C10-CPA) was found to be the strongest inhibitor, and its concentrations required for half-maximal inhibition for lasB-lacZ and rhlA-lacZ expression were 80 and 90 μM, respectively. C10-CPA also inhibited production of virulence factors, including elastase, pyocyanin, and rhamnolipid, and biofilm formation without affecting growth of P. aeruginosa PAO1. C10-CPA inhibited induction of both lasI-lacZ by N-(3-oxododecanoyl)-l-homoserine lactone (PAI1) and rhlA-lacZ by N-butanoyl-l-homoserine lactone (PAI2) in the lasI rhlI mutant of P. aeruginosa PAO1, indicating that C10-CPA interferes with the las and rhl quorum-sensing systems via inhibiting interaction between their response regulators (LasR and RhlR) and autoinducers.
Pseudomonas aeruginosa is a gram-negative bacterium capable of infecting insects, plants, animals, and humans (29). It is one of the most frequently isolated bacteria in nosocomial infections (6). As an opportunistic human pathogen, it colonizes immunocompromised hosts and mechanically ventilated patients. Most notably, cystic fibrosis patients are particularly susceptible to chronic infections by P. aeruginosa, which is a leading cause of mortality in this population (13). The capacity of this bacterium to cause such diverse infections is due to the production of a plethora of virulence factors (38).
It has been shown that P. aeruginosa employs quorum sensing in the regulation of genes encoding extracellular virulence factors (35). Quorum sensing is an intercellular communication system that allows bacteria to control gene expression in a cell population density-dependent manner. P. aeruginosa possesses two quorum-sensing systems termed las and rhl. The las and rhl quorum-sensing systems are comprised of the LuxRI homologs LasRI and RhlRI, respectively (9). The LuxI homologs LasI and RhlI are responsible for the synthesis of the las and rhl signals, N-(3-oxododecanoyl)-l-homoserine lactone (PAI1) (Fig. 1) and N-butanoyl-l-homoserine lactone (PAI2; Fig. 1), respectively. PAI1 activates the LuxR-type transcription factor LasR, and in turn LasR-PAI1 induces the production of LasB elastase, LasA protease, alkaline protease, exotoxin A, and LasRI (10, 11, 32, 37). The las quorum-sensing system is also required for the development of P. aeruginosa biofilms (5). PAI2 cooperates with the cognate regulator RhlR to enhance the production of rhamnolipid, pyocyanin, LasB elastase, hydrogen cyanide, and cytotoxic lectins (1, 20, 25, 41). The las quorum-sensing system regulates the rhl quorum-sensing system at the transcriptional and posttranscriptional levels, indicating these systems comprise a hierarchical cascade (28).
FIG. 1.
Effects of N-acyl-cyclopentylamides on lasB-lacZ expression in P. aeruginosa PAO1 and chemical structures of PAI1, PAI2, and C10-CPA. N-Acyl-pentylamides with acy side chain lengths ranging from C4 to C12 are abbreviated C4-CPA to C12-CPA. PAO1(pβ01) was cultivated for 24 h in the presence of 250 μM N-acyl-cyclopentylamide. Then β-galactosidase activity was determined. Horizontal bars represent the standard deviations of measurements done in four separate experiments. Different letters on the right sides of the bars indicate a significant difference (P < 0.01) among the treatments.
Because expression of many virulence factors and establishment of chronic infections are regulated by quorum sensing and disruption of quorum sensing resulted in reduced virulence in P. aeruginosa (4, 27, 36), quorum-sensing inhibitors are expected to reduce the pathogenicity of P. aeruginosa. Several small molecules have been identified as quorum-sensing inhibitors and have been shown to inhibit production of P. aeruginosa virulence factors regulated by quorum sensing. Smith et al. (33, 34) synthesized a library of PAI1 and PAI2 analogs with the homoserine lactone moiety replaced with different alcohols and amines and screened the library for antagonists that interfere with quorum sensing. They found that N-(2-oxocyclohexyl)-3-oxododecanamide had antagonist activity toward the las quorum-sensing system. N-(2-Hydroxyphenyl)-3-oxododecanamide was shown to interfere with the rhl quorum-sensing system as well as the las quorum-sensing system. The Australian marine macroalga Delisea pulchra has been known to produce halogenated furanones that antagonize bacterial quorum sensing (12). Hentzer et al. (14, 15) used the natural antagonists as a starting point for antagonist design and demonstrated that the synthetic furanone compound 4-bromo-5-(bromomethylene)-2(5H)-furanone specifically targeted quorum-sensing systems and inhibited virulence factor expression.
We have synthesized autoinducer analogs to find agonists and antagonists for bacterial quorum sensing. In the previous study, we synthesized optically pure enantiomers of N-butanoyl homoserine lactones and thiolactones (18). Bioassays revealed that l-isomers of N-butanoyl homoserine thiolactone acted as an autoinducer for quorum sensing in P. aeruginosa, while no effects were observed with d-isomers of N-buotanoyl homoserine lactone and thiolactone. In the course of the research, we observed that N-octanoyl cyclopentylamide (C8-CPA) moderately inhibited expression of the lasB-lacZ transcriptional fusion gene in P. aeruginosa PAO1. In the present study, we set C8-CPA as a lead compound and synthesized a series of structural analogs of C8-CPA to obtain stronger quorum-sensing inhibitors. We demonstrate that N-decanoyl cyclopentylamide (C10-CPA; Fig. 1) is a much stronger quorum-sensing inhibitor that interferes with expression of P. aeruginosa virulence factors regulated by the las and rhl quorum-sensing systems.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. P. aeruginosa PAO-NN1 was provided by Nobuhiko Nomura, Tsukuba University. P. aeruginosa and Escherichia coli strains were grown at 37°C with shaking in LB medium (30) supplemented with the appropriate antibiotics. Pseudomonas broth (7) was used for production of pyocyanin. Autoinducers and their analogs were added to medium at the start of cultivation.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| MV1184 | ara-14 Δ(lac-proAB) rpsL thi-1 (φ80 lacZΔM15) Δ(srl-recA)360::Tn10 F′[traD36 proAB+lacIqlacZΔM15] | 39 |
| Pseudomonas aeruginosa | ||
| PAO1 | Prototroph | 17 |
| PAO-MW1 | lasI rhlI double mutant of PAO1 | 40 |
| PAO-NN1 | lasR mutant of PAO1 | N. Nomura |
| Plasmids | ||
| pQF50 | Broad-host-range transcriptional fusion vector; CbrlacZ IncP | 8 |
| pβ01 | pQF50 derivative containing lasB-lacZ transcriptional fusion | 18 |
| pβ02 | pQF50 derivative containing rhlA-lacZ transcriptional fusion | 18 |
| pβ03 | pQF50 derivative containing lasI-lacZ transcriptional fusion | This study |
| pMRP9-1 | Broad-host-range GFP expression vector; Cbr | 23 |
Cbr, carbenicillin resistant.
DNA manipulation and electroporation.
Standard procedures were used for plasmid DNA preparations, restriction enzyme digestions, ligations, transformations, and agarose gel electrophoresis (30). PCRs were carried out using KOD plus DNA polymerase (Toyobo, Tokyo, Japan) according to the manufacturer's instructions. P. aeruginosa was transformed by electroporation as described previously (21).
Plasmid construction.
The promoter region of lasI was amplified from the P. aeruginosa PAO1 genome by PCR with oligonucleotides 5′-GCCCGGAAGGCCATGTTTTG-3′ and 5′-GGGCCAGTGGTATCGAGAAT-3′. The PCR product was cloned into the SmaI site of pQF50 to construct pβ03.
Synthesis of autoinducer analogues.
Acyl cycloalkylamides were synthesized by using appropriate acyl chlorides and cycloalkylamines. Appropriate acyl chloride (1 mmol) was added to 2 ml of dry dichloromethane containing 2 mmol of cycloalkylamine. The mixture was stirred for 4 to 6 h. After removing dichloromethane by evaporation, the residue was dissolved into 20 ml diethyl ether and washed with water, 5% NaHCO3, 0.2 M HCl, and saturated NaCl solution. The organic extracts were dried over MgSO4 and concentrated to give acyl cycloalkylamines. N-Decanoyl-l-homoserine lactone was synthesized by using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide in an aqueous solution as previously described (18). PAI1 was synthesized as described by Chhabra et al. (2). The purities of all synthesized compounds were confirmed by elemental analysis and 500 MHz 1H- and 125 MHz 13C-nuclear magnetic resonance spectra.
Enzyme assays.
For β-galactosidase measurements, P. aeruginosa lasB-lacZ and rhlA-lacZ reporter strains were grown with shaking in LB medium at 37°C for 24 h. The lasI-lacZ reporter strain was cultivated for 9 h because lasI is one of the early quorum-sensing-controlled genes which show activation upon addition of acyl homoserine lactone signals even in early logarithmic phase (32). β-Galactosidase activities of P. aeruginosa cells were determined as described by Miller (22), with the modification that the enzymatic reaction was carried out at 37°C. Elastolytic activity in culture supernatants was determined by elastin-Congo red assays (26) with modifications. Cells were grown with shaking in LB medium at 37°C for 24 h. One milliliter of culture supernatants was added to tubes containing 10 mg of elastin-Congo red (Sigma) and 2 ml of buffer A (0.1 M Tris-HCl [pH 7.0], 1 mM CaCl2). Tubes were incubated for 2 h at 37°C with shaking. The reaction was terminated by the addition of 2 ml of sodium phosphate buffer (0.7 M [pH 6.0]). The precipitate was removed by centrifugation, and the absorption was measured at 495 nm.
Assays for rhamnolipid and pyocyanin production.
Rhamnolipid in P. aeruginosa culture fluids was extracted and determined by orcinol assays (19) as previously described (26). Pyocyanin was extracted from culture supernatants and measured as previously described (7). Three milliliters of chloroform was mixed with 5 ml culture supernatant. The chloroform layer was transferred to a fresh tube and mixed with 1 ml of 0.2 M HCl. After centrifugation, the top layer was removed and its absorption was measured at 520 nm.
Visualization and analysis of P. aeruginosa biofilms.
Biofilms were grown in flow cells (channel dimensions, 3 by 3.2 by 40 mm; flow rate, 170 μl min−1) with modified FAB medium (16). The once-through continuous-culture biofilm reactor system was assembled and prepared as described by Christensen et al. (3). The channel was inoculated with overnight cultures of P. aeruginosa PAO1(pMRP9-1) and incubated without flow for 2 h at room temperature, then medium flow was reinitiated in order to wash free, nonadherent cells out of the system. Bacterial cells were visualized with a scanning confocal laser microscope (model Fluoview; Olympus, Tokyo, Japan).
Statistical analysis.
Statistical comparisons were conducted with analysis of variance followed by Tukey's honest significant difference post hoc pairwise comparisons using Statcel 2 (OMS Press, Saitama, Japan).
RESULTS AND DISCUSSION
The lasB-lacZ reporter assays of C8-CPA structural analogs.
In the previous study, we tested PAI2 analogs with substitutions within the l-homoserine lactone ring for effects on quorum sensing in P. aeruginosa and found that conversion of the l-homoserine lactone ring to an l-homocysteine thiolactone ring did not affect the ability of the molecule to act as an autoinducer and that the d-isomer of PAI2 showed neither agonistic nor antagonistic effects (18). Subsequently, we examined acyl homoserine lactone analogs with various ring structures and acyl chain lengths and found that C8-CPA had a moderate inhibitory effect on the induction of the quorum-sensing-controlled gene lasB, which encodes LasB elastase. Passador et al. (24) synthesized a series of structural analogs of P. aeruginosa autoinducer PAI1 and examined them for their ability to act as autoinducer agonists and antagonists. They demonstrated that the length of the acyl side chain of the autoinducer molecule is the critical factor for agonistic and antagonistic activities. Therefore, it is possible that the length of the acyl side chain of N-acyl-cyclopentylamides (acyl-CPAs) has effects on their ability to inhibit expression of quorum-sensing-controlled genes. To assess this possibility, a series of acyl-CPAs with acyl chain lengths ranging from C4 to C12 were synthesized and tested for their ability to inhibit expression of a lasB-lacZ transcriptional fusion using the P. aeruginosa PAO1(pβ01) reporter strain. C8-CPA exhibited a significant inhibitory effect on lasB-lacZ expression (P < 0.01) (Fig. 1). The lasB-lacZ reporter assays revealed that C10-CPA had a significant stronger inhibitory effect than C8-CPA (P < 0.01) and inhibited lasB-lacZ expression 80% at 250 μM.
We then synthesized C10-CPA structural analogs to investigate effects of the ring structure on inhibitory activity and examined their ability to act as inhibitors. C10-CPA analogs with cyclopropylamide, cyclobutylamide, cyclohexylamide, cycloheptylamide, and cyclooctylamide rings demonstrated little inhibitory activity (data not shown). N-Decanoyl-l-homoserine lactone (C10-HSL), which is a C10-CPA analog with the l-homoserine lactone ring, also failed to show inhibitory action in the lasB-lacZ reporter assay (data not shown). These results suggest that not only the length of the side acyl chain but also the ring structure is critical for C10-CPA to act as an inhibitor. Since C10-CPA was found to be the most active inhibitor of synthesized analogs, C10-CPA was used for further study.
Inhibitory activity of C10-CPA.
Figure 2 shows the time course of growth and lasB-lacZ expression when P. aeruginosa PAO1(pβ01) was cultivated in the presence or in the absence of 250 μM C10-CPA. C10-CPA inhibited lasB-lacZ expression in P. aeruginosa PAO1 without affecting growth. The lasB-lacZ reporter assays were performed at a range of concentrations of C10-CPA. The result revealed that C10-CPA significantly inhibited lasB-lacZ expression in a dose-dependent manner (Fig. 3A). The concentration of C10-CPA required for half-maximal inhibition (IC50) was approximately 80 μM.
FIG. 2.
Time course of cell growth (open symbols) and lasB-lacZ expression (closed symbols). P. aeruginosa PAO1(pβ01) was cultivated in LB medium with no added C10-CPA (open and closed circles) and 250 μM C10-CPA (open and closed squares). OD600, optical density at 600 nm.
FIG. 3.
Dose response for inhibitory effects of C10-CPA on lasB-lacZ (A) and rhlA-lacZ (B) expression in P. aeruginosa PAO1. PAO1(pβ01) and PAO1(pβ02) cells were cultivated for 24 h with a range of concentrations of C10-CPA, and β-galactosidase activity was measured. Vertical bars represent the standard deviations of measurements done in four separate experiments. Different letters above bars indicate a significant difference (P < 0.01) among the treatments.
We then investigated effects of C10-CPA on another quorum-sensing-controlled gene, rhlA. Plasmid pβ02 carrying the rhlA-lacZ transcriptional fusion gene was introduced into the wild-type PAO1 strain, and the resulting PAO1(pβ02) strain was used as the rhlA-lacZ reporter strain. C10-CPA also significantly inhibited rhlA-lacZ expression in P. aeruginosa PAO1 in a dose-dependent manner (Fig. 3B). The IC50 for rhlA-lacZ expression was about 90 μM. It is interesting to note that other C10-CPA analogs including C10-HSL had little or no inhibitory effect on rhlA-lacZ expression (data not shown).
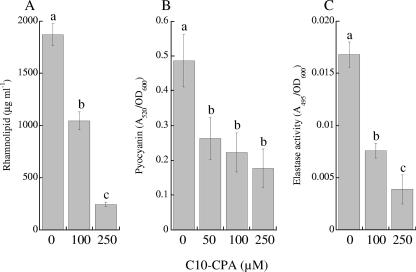
To further confirm that C10-CPA is disrupting quorum sensing, we examined C10-CPA for its ability to reduce quorum-sensing-controlled virulence factors. P. aeruginosa PAO1 cells were grown with and without C10-CPA for 24 h, and levels of elastase activity, pyocyanin, and rhamnolipid in culture supernatants were determined. Production of rhamnolipid and elastase was significantly inhibited by C10-CPA in a dose-dependent manner (Fig. 4). Inhibition of pyocyanin production by C10-CPA was also significant but not dose dependent. Elastase, rhamnolipid, and pyocyanin were reduced to 23%, 13%, and 36% of the control levels, respectively, in the presence of 250 μM C10-CPA.
FIG. 4.
Inhibitory effects of C10-CPA on pyocyanin (A), rhamnolipid (B), and elastase (C) production by P. aeruginosa PAO1. PAO1 was cultivated for 24 h without C10-CPA or with 50 to 250 μM C10-CPA. Then concentrations of pyocyanin and rhamnolipid and elastase activity in culture supernatants were measured. Vertical bars represent the standard deviations of measurements done in four separate experiments. Different letters above bars indicate a significant difference (P < 0.01) among the treatments.
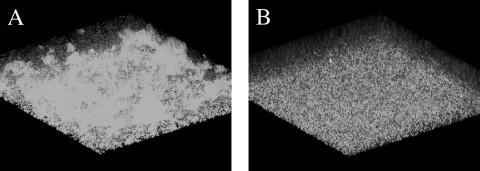
There is increasing evidence that quorum sensing plays a crucial role in the maturation of P. aeruginosa biofilms. Therefore, we examined the effect of C10-CPA on biofilm formation in P. aeruginosa. The wild-type strain PAO1, carrying the green fluorescent protein gene (gfp) expression vector pMRP9-1, was cultivated in flow cells for 1 week in the absence or in the presence of 250 μM C10-CPA, and scanning confocal photomicrographs were captured. As shown in Fig. 5, PAO1(pMRP9-1) formed mature biofilms several hundred micrometers thick in the absence of C10-CPA. However, the same strain did not form biofilm even after 1 week of cultivation in the presence of C10-CPA.
FIG. 5.
Effect of C10-CPA on wild-type P. aeruginosa biofilm formation. PAO1 carrying the gfp expression vector pMRP9-1 was cultivated in flow cells in the absence (A) or presence (B) of 250 μM C10-CPA.
The transcription of the gfp gene carried on pMRP9-1 is controlled by the lac promoter (23). C10-CPA did not affect GFP expression in PAO1(pMRP9-1) at 250 μM (data not shown). Moreover, the growth of PAO1 was not affected by the addition of C10-CPA even at concentrations up to 250 μM (Fig. 2). These results indicate that C10-CPA specifically interferes with the quorum-sensing systems.
Target of inhibitory action by C10-CPA.
Davies et al. (5) reported that the las system, but not the rhl system, is required for the ability to form biofilms in flow chamber systems. Previous studies demonstrated that lasB (LasB elastase gene), rhlAB (rhamnosyltransferase genes), and phzABCDEFG (pyocyanin synthesis genes) responded to the las and rhl systems and that their maximal expression required both systems (31, 40). The rhlI and rhlR genes, which encode the PAI2 synthase and the response regulator in the rhl system, are positively regulated by the las system (28). The las system employs PAI1 as an autoinducer, which binds to LasR, and LasR-PAI1 complex stimulates expression of target genes. These facts and results obtained in this study allow us to speculate that the autoinducer analog C10-CPA interferes with the las system, possibly via acting as an effective competitor of autoinducer binding to LasR.
To assess the possibilities, we tested effects of C10-CPA on lasI expression which is regulated by only the las system (26). The lasI promoter region was cloned to pQF50 to construct lasI-lacZ reporter plasmid pβ03. P. aeruginosa PAO1 harboring pβ03 was examined for lasI-lacZ expression. As expected, C10-CPA significantly inhibited lasI-lacZ expression in a dose-dependent manner (Fig. 6). We then introduced pβ03 to lasI rhlI double-mutant P. aeruginosa PAO-MW1, which does not produce PAI1 or PAI2. PAO-MW1(pβ03) showed only the basal level of β-galactosidase (Fig. 6). Transcription of lasI-lacZ increased when PAI1 was exogenously added. C10-CPA significantly inhibited this induction of lasI-lacZ in PAO-MW1(pβ03). This result demonstrated that C10-CPA interferes with the las system via inhibiting LasR-PAI1 interaction.
FIG. 6.
(A) Dose response for inhibitory effects of C10-CPA on lasI-lacZ expression in P. aeruginosa PAO1. (B) Inhibition of PAI1 signaling by C10-CPA. The LasI-RhlI signal mutant PAO-MW1 harboring pβ03 was grown in the presence of and in the absence of 10 nM PAI1 and 250 μM C10-CPA for 9 h, and β-galactosidase activity was measured. Vertical bars represent the standard deviations of measurements done in four separate experiments. Different letters above bars indicate a significant difference (P < 0.01) among the treatments.
We then investigated whether C10-CPA inhibits the rhl system or not. Since the rhlA promoter is regulated by both las and rhl systems, the rhlA-lacZ reporter assays were conducted in the LasR-deficient background to test effects of C10-CPA on the rhl system. The rhlA-lacZ reporter plasmid pβ02 was introduced into lasR mutant P. aeruginosa PAO-NN1, and the resulting PAO-NN1(pβ02) strain was used as the reporter strain. The reporter assays revealed that C10-CPA significantly inhibited rhlA-lacZ expression in PAO-NN1(pβ02) (Fig. 7A), suggesting that C10-CPA has an inhibitory effect on the rhl system. We speculated that C10-CPA interfered with the rhl system via inhibiting RhlR-PAI2 interaction. To address this speculation, the rhlA-lacZ reporter assays were carried out using PAO-MW1(pβ02) as the reporter strain. PAO-MW1(pβ02) showed the basal level of β-galactosidase due to the loss of PAI1 and PAI2 production, and the exogenous addition of PAI2 increased rhlA-lacZ expression (Fig. 7B). C10-CPA significantly inhibited the induction of rhlA-lacZ by PAI2 in PAO-MW1(pβ02). Since PAI2 does not activate LasR (24), these results indicate that C10-CPA also interferes with the rhl system via inhibiting RhlR-PAI2 interaction.
FIG. 7.
(A) Dose response for inhibitory effects of C10-CPA on rhlA-lacZ expression in P. aeruginosa PAO-NN1. (B) Inhibition of PAI2 signaling by C10-CPA. The LasI-RhlI signal mutant PAO-MW1 harboring pβ02 was grown in the presence of and in the absence of 2 μM PAI2 and 250 μM C10-CPA for 24 h, and β-galactosidase activity was measured. Vertical bars represent the standard deviations of measurements done in four separate experiments. Different letters above bars indicate a significant difference (P < 0.01) among the treatments.
In summary, C10-CPA was found to have strong inhibitory effects on expression of quorum-sensing-controlled genes and virulence factors in P. aeruginosa. The length of the acyl side chain and the ring structure are critical for the inhibitory activity of C10-CPA. C10-CPA inhibited production of elastase, pyocyanin, and rhamnolipid and biofilm formation. C10-CPA intervenes with the las and rhl system via inhibiting interaction between their response regulators and autoinducers.
Acknowledgments
We thank Nobuhiko Nomura (Tsukuba University, Ibaragi, Japan) for providing P. aeruginosa PAO-NN1.
This study was in part supported by a grant for scientific research from the Ministry of Education, Science and Culture of Japan.
Footnotes
Published ahead of print on 16 March 2007.
REFERENCES
- 1.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhabra, S. R., C. Harty, D. S. Hooi, M. Daykin, P. Williams, G. Telford, D. I. Pritchard, and B. W. Bycroft. 2003. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-l-homoserine lactone as immune modulators. J. Med. Chem. 46:97-104. [DOI] [PubMed] [Google Scholar]
- 3.Christensen, B., B. C. Sternberg, J. B. Andersen, R. J. Palmer, A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 4.Darby, C., C. L. Cosma, J. H. Thomas, and C. Manoil. 1999. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:15202-15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 6.Emori, T. G., and R. P. Gaynes. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Essar, D. W., L. Eberly, A. Hadero, and I. P. Crawford. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 172:884-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farinha, M. A., and A. M. Kropinski. 1990. Construction of broad-host-range plasmid vector for easy visible selection and analysis of promoters. J. Bacteriol. 172:3496-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum sensing transcriptional regulators. Annu. Microbiol. Rev. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 10.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambello, M. J., S. Kaye, and B. H. Iglewski. 1993. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 61:1180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hentzer, M., K. Riedel, T. B. Rasmussen, A. Heydorn, J. B. Andersen, M. R. Parsek, S. A. Rice, L. Eberl, S. Molin, N. Høiby, S. Kjelleberg, and M. Givskov. 2002. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148:87-102. [DOI] [PubMed] [Google Scholar]
- 15.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Høiby, and M. Givskov. 2005. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 17.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda, T., K. Kajiyama, T. Kita, N. Takiguchi, A. Kuroda, J. Kato, and H. Otake. 2001. The synthesis of optically pure enantiomers of N-acyl-homoserine lactone autoinducers and their analogues. Chem. Lett. 2001:314-315. [Google Scholar]
- 19.Koch, A. K., O. Käppeli, A. Fiechter, and J. Reiser. 1991. Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J. Bacteriol. 173:4212-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. A. B. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 21.Masduki, A., J. Nakamura, T. Ohga, R. Umezaki, J. Kato, and H. Ohtake. 1995. Isolation and characterization of chemotaxis mutants and genes of Pseudomonas aeruginosa. J. Bacteriol. 177:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 23.Parsek, M. R., and E. P. Greenberg. 1999. Quorum sensing signals in development of Pseudomonas aeruginosa biofilms. Methods Enzymol. 310:43-55. [DOI] [PubMed] [Google Scholar]
- 24.Passador, L., K. D. Tucker, K. R. Guertin, M. P. Journet, A. S. Kende, and B. H. Iglewski. 1996. Functional analysis of the Pseudomonas aeruginosa autoinducer PAI. J. Bacteriol. 178:5995-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson, J. P., M. Feldman, B. H. Iglewski, and A. Prince. 2000. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68:4331-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1850. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seed, P. C., L. Passador, and B. H. Iglewski. 1995. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, K. M., Y. Bu, and H. Suga. 2003. Induction and inhibition of Pseudomonas aeruginsa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 10:81-89. [DOI] [PubMed] [Google Scholar]
- 34.Smith, K. M., Y. Bu, and H. Suga. 2003. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginsa autoinducer. Chem. Biol. 10:563-571. [DOI] [PubMed] [Google Scholar]
- 35.Swift, S., J. A. Downie, N. A. Whitehead, A. M. L. Barnard, G. P. C. Salmond, and P. Williams. 2001. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microbiol. Physiol. 45:199-270. [DOI] [PubMed] [Google Scholar]
- 36.Tan, M. W., L. G. Rahme, J. A. Sternberg, R. G. Tompkins, and F. M. Ausubel. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 96:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toder, D. S., M. J. Gambello, and B. H. Iglewski. 1991. Pseudomonas aeruginosa LasA—a 2nd elastase under the transcriptional control of LasR. Mol. Microbiol. 5:2003-2010. [DOI] [PubMed] [Google Scholar]
- 38.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 40.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winzer, K., C. Falconer, N. C. Garber, S. P. Diggle, M. Camara, and P. Williams. 2000. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J. Bacteriol. 182:6401-6411. [DOI] [PMC free article] [PubMed] [Google Scholar]