Abstract
VanA-type human (n = 69), animal (n = 49), and food (n = 36) glycopeptide-resistant enterococci (GRE) from different geographic areas were investigated to study their possible reservoirs and transmission routes. Pulsed-field gel electrophoresis (PFGE) revealed two small genetically related clusters, M39 (n = 4) and M49 (n = 13), representing Enterococcus faecium isolates from animal and human feces and from clinical and fecal human samples. Multilocus sequence typing showed that both belonged to the epidemic lineage of CC17. purK allele analysis of 28 selected isolates revealed that type 1 was prevalent in human strains (8/11) and types 6 and 3 (14/15) were prevalent in poultry (animals and meat). One hundred and five of the 154 VanA GRE isolates, encompassing different species, origins, and PFGE types, were examined for Tn1546 type and location (plasmid or chromosome) and the incidence of virulence determinants. Hybridization of S1- and I-CeuI-digested total DNA revealed a plasmid location in 98% of the isolates. Human intestinal and animal E. faecium isolates bore large (>150 kb) vanA plasmids. Results of PCR-restriction fragment length polymorphism and sequencing showed the presence of prototype Tn1546 in 80% of strains and the G-to-T mutation at position 8234 in three human intestinal and two pork E. faecium isolates. There were no significant associations (P > 0.5) between Tn1546 type and GRE source or enterococcal species. Virulence determinants were detected in all reservoirs but were significantly more frequent (P < 0.02) among clinical strains. Multiple determinants were found in clinical and meat Enterococcus faecalis isolates. The presence of indistinguishable vanA elements (mostly plasmid borne) and virulence determinants in different species and PFGE-diverse populations in the presence of host-specific purK housekeeping genes suggested that all GRE might be potential reservoirs of resistance determinants and virulence traits transferable to human-adapted clusters.
Enterococci are gram-positive, opportunistic bacteria that inhabit the gastrointestinal tracts of humans and many animals. They are also present in food, as starter cultures for the production of cheese and fermented sausages or as fecal contaminants of raw meat, milk, and milk products. Some specific strains are available as probiotics in animal feeds (20, 22). However, enterococci have gained notoriety as a major cause of nosocomial infection and are increasingly isolated from the bloodstream, urinary tract, and surgical sites. Enterococcus faecalis causes 80 to 90% of human enterococcal infections, and Enterococcus faecium causes most of the remaining cases (other enterococcal species being infrequently involved) (27). The emergence of multidrug resistance (i.e., resistance to multiple antibacterial agents), including high-level resistance to glycopeptides, among enterococci, particularly E. faecium, has resulted in clinical isolates resistant to all antibiotics of proven efficacy (7). Glycopeptide-resistant enterococci (GRE), which have emerged as nosocomial pathogens in the past 10 to 15 years (7), are a global problem despite major epidemiological differences between Europe and the United States. A high frequency of GRE has been reported in hospitals in the United States, whereas extremely low frequencies have been reported in the community, in animals, and in food of animal origin (7, 12, 29, 39, 51).
Results of studies conducted in northern European countries have revealed a low overall prevalence of GRE infection in Europe, with GRE being detected mostly in the nonhospitalized healthy population and among animals (7, 51). However, their incidence in clinical infections has been rising in southern Europe (Portugal, Greece, and Italy) and, though remaining generally lower that those described in the United States (European Antimicrobial Resistance Surveillance System, http://www.earss.rivm.nl, last accessed 10 October 2005), rates of 20% for clinical infections and of 7.5% for intestinal colonization in at-risk hospital wards have been reported in Italy (25).
The spread of GRE was linked to the use of the glycopeptide avoparcin as a growth promoter in animal husbandry (37, 51), until its ban in the European Union in 1997. Despite indirect evidence for dissemination to humans of glycopeptide resistance selected in animals by clonal spread or horizontal resistance gene transfer (51), in Italy and other southern European countries GRE isolation has so far been reported mostly in hospital settings (7, 25; http://www.earss.rivm.nl), whereas relatively few data are available with regard to isolation in the community and nonhuman sources (5, 7, 15).
The VanA phenotype, expressing inducible, high-level vancomycin and teicoplanin resistance, is the most common in Europe (3, 5, 25, 29, 37). The vanA cluster—detected primarily in E. faecium and E. faecalis and less frequently in other enterococcal species—is carried by Tn1546 and is transferable by conjugation (2, 9). Considerable heterogeneity may exist among Tn1546 elements, largely resulting from the presence of insertion sequences or from deletions in nonessential genes and intergenic regions (32, 51, 57).
The pathogenesis of enterococcal infections is still poorly understood, although several virulence factors, such as aggregation substance(s) (AS), gelatinase (Gel), cytolysin (Cyl), and enterococcal surface protein (Esp), have been described (24, 27). AS are pheromone-inducible surface proteins of E. faecalis that facilitate the conjugative exchange of plasmids (carrying virulence and/or antibiotic resistance genes) and also contribute to pathogenicity by enhancing adhesion to and internalization by cultured human cells, as well as favoring intracellular survival within macrophages (10, 52, 55). Although sex pheromone plasmids are highly specific for E. faecalis, they have also been detected in vancomycin-resistant E. faecium strains (28, 40). Gel, a secreted Zn metalloprotease, and Cyl, a lytic toxin, have been implicated in the pathogenicity of E. faecalis on the basis of both epidemiological data and studies of animal models (24, 27, 48). Esp is a surface protein involved in the ability to colonize and in immune evasion in E. faecalis and E. faecium (21, 24). Enterococci are also known to produce slime (17, 18) and to form biofilms, which have been regarded as virulence features of clinical isolates (16, 18).
Several reviews have addressed the genetic basis, reservoirs, and spread of glycopeptide resistance in enterococci (7, 9, 51) and enterococcal virulence (24, 27, 47). A combination of glycopeptide resistance and virulence in enterococci could pose a serious threat to human health. However, data on the presence of virulence traits in GRE are scarce (42, 44).
The present study was undertaken to explore the relatedness of GRE of different origins (human, animal, and food) and from different geographic areas to gain a better understanding of the involvement of the different reservoirs in the emergence and spread of virulent clones, i.e., those that in addition to antibiotic resistance have also acquired a number of genes conferring infectivity and virulence. To do this, human, animal, and food GRE were analyzed for population structure (using pulsed-field gel electrophoresis [PFGE], purK allele sequence analysis, and multilocus sequence typing [MLST]) and Tn1546 type and location (chromosome or plasmid) as well as for the presence and expression of the main virulence determinants.
MATERIALS AND METHODS
Bacterial strains and media.
We studied a total of 154 VanA-type GRE from humans (n = 69), animals (n = 49), and food (n = 36). See Table 1 for details. Most of the strains (n = 142) were collected throughout Italy between 1997 and 2003 (64 human, 42 animal, and 36 food isolates). The 11 non-Italian GRE isolates were from Belgium (n = 1) and Norway (n = 10); of these, five were human intestinal and six were animal isolates. Reference and control strains are listed in Table 2.
TABLE 1.
Strains collected in this study
| Strain and origin (n) | Source (n) | Area | Yr | Isolate(s) |
|---|---|---|---|---|
| Italian strains | ||||
| Human intestinal (43) | Inpatientsa (37) | North | 1997 | VI1, VI46 |
| 2002 | MI24, MI25, MI26, MI28, MI29, MI30, MI31, MI32, MI33, MI34, MI54, MI55, MI56, MI57, MI58, MI59, MI60 | |||
| Center | 1997 | AN8 | ||
| 2002 | AN7, AN9, AN12, AN13, AN14, AN15, AN16, AN17, AN19, AN20, AN23, AN47, AN48, AN49, AN50, AN51, AN52 | |||
| Outpatients (6) | North | 2002 | MI27 | |
| Center | 2002 | AN10, AN11, AN18 | ||
| 2003 | AN53, AN61 | |||
| Animal (43) | Poultry (38) | North | 1997 | PD1, VI2, PD3, VI4, VR5, PD6, PD7, VR8, VI9, VI10, VI11, VR12, PD13, PD33, PD34, PD35, PD36, PD37, PD38, PD39, PD40, PD41, PD42 |
| 2001 | VE31, VE44, VE45, VE46, VE47, VE50 | |||
| Center | 2003 | AN14, AN25, AN26, AN27, AN28, AN29, AN30, AN43, AN49 | ||
| Pig (5) | Center | 2003 | AN21, AN22, AN23, AN24, PG15 | |
| Food (36) | Poultry meat (27) | North | 1998 | PM3, PM12, PM13 |
| 2000 | PM14, PM15, PM16, PM17, PM18, PM19, PM20, PM21, PM22, PM23, PM24, PM25, PM31, PM32 | |||
| 2001 | PM26, PM27, PM28, PM33 | |||
| Center | 1998 | PM10, PM11 | ||
| 2003 | PM2, PM30, PM35, PM36 | |||
| Pork meat (7) | Center | 2003 | KM1 | |
| South | 1998 | KM4, KM5, KM6, KM7, KM8, KM9 | ||
| Cheese (2) | North | 2003 | CH29, CH34 | |
| Human clinical (21) | Blood (10) | North | 1997 | VI4, MI66 |
| 1998 | MI65 | |||
| Center | 1998 | R35, AN64 | ||
| 2002 | R36, R39, R40 | |||
| 2003 | AN113, AN338 | |||
| Urine (9) | North | 1997 | VI2, VI3, UD5 | |
| 2001 | GE69 | |||
| Center | 1997 | AN21, AN67 | ||
| 1998 | AN22 | |||
| 2002 | R38, AN68 | |||
| Wound (1) | Center | 2002 | R37 | |
| Bile (1) | North | 1997 | UD6 | |
| Non-Italian strains | ||||
| Human intestinal (5) | Community | Norway | 1998 | HI-N41 to HI-N45 (31) |
| Animal (6) | Poultry | Norway | 1998 | A-N16 to A-N20 (31) |
| Belgium | 1997 | FAIR-E-16210 (EU-FAIR project CT97-3078) |
No enterococcal infection.
TABLE 2.
Control strains used in the study
| Strain | Purpose | Reference or source |
|---|---|---|
| E. faecium BM4147 | Glycopeptide resistance genotype, Tn1546 typing, probe synthesis and ddl PCR; vanA control strain | 38 |
| E. faecalis V583 | Glycopeptide resistance genotype, ddl PCR; vanB control strain | 45 |
| E. gallinarum ATCC 49573 | Glycopeptide resistance genotype, ddl PCR; vanC-1 control strain | ATCC |
| E. casseliflavus ATCC 14432 | Glycopeptide resistance genotype, ddl PCR; vanC-2 control strain | ATCC |
| E. durans ATCC 19432 | ddl PCR | ATCC |
| E. faecalis OG1RF(pCF10) | Clumping, aggregation substance identification; sex pheromone plasmid-harboring strain | 10 |
| E. faecalis OG1RF(pAD1) | Clumping, aggregation substance identification; sex pheromone plasmid-harboring strain | 10 |
| E. faecalis JH2-2 | Sex pheromone production | 40 |
| E. faecalis OG1RF | Sex pheromone production | 40 |
GRE were isolated on Slanetz-Bartley agar (Becton Dickinson, Milan, Italy) containing 6 μg/ml vancomycin. Fecal and meat homogenates were previously enriched in selective tryptone soy broth (Oxoid, Basingstoke, United Kingdom) containing 0.4 mg/ml sodium azide and 6 μg/ml vancomycin. Brain heart infusion broth and agar (Oxoid) were used for routine culture. Tryptone soy broth supplemented with 1% glucose was used for slime production assays. Gelatin infusion broth containing 40 mg/ml gelatin (Bio-Rad Laboratories, Richmond, CA) was used to determine gelatinase production. Blood agar base (Oxoid) supplemented with fresh horse blood (5%) was used to investigate hemolysin production. Isolates were maintained in glycerol at −70°C and subcultured twice on Slanetz-Bartley agar before testing.
Species identification and PFGE typing.
GRE were identified at the species level with API ID32-STREP kits (bioMérieux Italia, Rome, Italy) and additional biochemical tests (22) and species-specific enterococcal ddl PCR performed with the primers listed in Table 3. PFGE of SmaI (New England Biolabs, Beverly, MA)-digested total DNA was performed essentially as described previously (3) using a CHEF Mapper apparatus (Bio-Rad) with pulse time increasing from 1 to 20 s over 20 h at 200 V (6 V/cm). Genetic relatedness was interpreted according to the method of Tenover et al. (53). Strains differing by six or fewer bands were grouped into the same PFGE type (1, 2, 3, etc.) and subdivided into PFGE subtypes (1a, 1b, 1c, etc.) based on single-band differences. PFGE data were analyzed separately for each species, considering each band as a separate putative locus and scoring it as present (1) or absent (0) in each accession. Dendrograms were constructed by the use of the Dice coefficient and the unweighted-pair group method using arithmetic averages.
TABLE 3.
PCR primers and products
| Purpose and primers | Gene target(s) | Primer sequence (5′-3′) | Positiona | Product length (bp) | Reference |
|---|---|---|---|---|---|
| Identification and 16S probe | |||||
| HiF | ddlE. hirae | TTATGTCCCTGTTTTGAAAAA | 485-506b | 378 | 36 |
| HiR | TTTTGATAGACCTCTTCCGGT | 868-845b | |||
| DuF | ddlE. durans | TTATGTCCCAGTATTGAAAAA | 485-506b | 189 | 36 |
| DuR | TGAATCATATTGGTATGCAGT | 649-672b | |||
| DDLM1 | ddlE. faecium | TAGAGACATTGAATATGCC | 359-377 | 528 | 19 |
| DDLM2 | CTAACATCGTGTAAGCT | 887-870 | |||
| DDLS1 | ddlE. faecalis | ATCAAGTACAGTTAGTCT | 98-116 | 941 | 19 |
| DDLS2 | ACGATTCAAAGCTAACTG | 1038-1021 | |||
| P1 | rRNA gene 16S | GCGGCGTGCCTAATACATGC | 39-58b | 957 | 35 |
| 16S1 | TGCATTAGCTAGTTGGTGAGG | 240-260b | 726 | 14 | |
| 16S2c | TCGAATTAAACCACATGCTCC | 964-944b | 14 | ||
| Glycopeptide resistance genotype | |||||
| VANA1 | vanA | GGGAAAACGACAATTGC | 175-191 | 732 | 19 |
| VANA2 | GTACAATGCGGCCGTTA | 907-891 | |||
| VANB | vanB | ATGGGAAGCCGATAGTC | 173-189 | 635 | 19 |
| VANB2 | GATTTGCTTCCTCGACC | 807-791 | |||
| VANC1-1 | vanC1 | GGTATCAAGGAAACCTC | 246-272 | 822 | 19 |
| VANC1-2 | CTTCCGCCATCATAGCT | 1067-1051 | |||
| VANC2/3-1 | vanC2/3 | CTCCTACGATTCTCTTG | 455-486 | 439 | 19 |
| VANC2/3-2 | CGAGCAAGACCTTTAAG | 885-869 | |||
| Tn1546 | |||||
| IR | Tn1546 | GGAAAATGCGGATTTACAACGCTAAG | 13-38, 10814-10839 | 10,826 | 43 |
| INV2 | orf2-vanX | ATGAGGTGATATTTTGCGGAAA | 3174-3195* | 5,405 | 23 |
| VANX2 | CTATTGGGGTATGGTTCGTCT | 8579-8599* | 4 | ||
| ORF1A | orf1 | AGGGCGACATATGGTGTAACA | 170-190* | 41 | |
| ORF1B | orf1 | TGGTGGCTCCTTTTCCCAGTTC | 907-928* | 41 | |
| ORF1C | orf1 | ACCGTTTTTGCAGTAAGTCTAAAT | 1871-1894* | 41 | |
| ORF2R | orf2 | TTTCCGCAAAATATCACCTCAT | 3195-3174* | This study | |
| INV3 | vanX-vanZ | AGACGAACCATACCCCAATAG | 8578-8596* | 1,999 | 23 |
| VANZ1 | GGTACGGTAAACGAGCAATAATA | 10577-10555* | 4 | ||
| Virulence factors | |||||
| AGG1 | prgB, asaI, aspI | AAGAAAAAGAAGTAGACCAAC | 601-622 | 1,555 | 20 |
| AGG2 | AAACGGCAAGACAAGTAAATA | 2156-2133 | |||
| ASA373F | asa373 | GGACGCACGTACACAAAGCTAC | 3094-3115 | 619 | 13 |
| ASA373R | CTGGGTGTGATTCCGCTGTTA | 3693-3713 | |||
| GELE1 | gelE | ACGCATTGCTTTTCCATC | 729-746 | 419 | 20 |
| GELE2 | ACCCCGTATCATTGGTTT | 1148-1129 | |||
| CYLB1 | cylB | ATTCCTACCTATGTTCTGTTA | 1199-1219 | 843 | 20 |
| CYLB2 | AATAAACTCTTCTTTTCCAAC | 2041-2021 | |||
| ESP1 | esp | TTGCTAATGCTAGTCCACGACC | 1217-1238 | 932 | 20 |
| ESP2 | GCGTCAACACTTGCATTGCCGA | 2149-2128 |
Positions are from the first base of the coding sequence, except for positions marked with asterisks, which are from the first base of IRL of Tn1546.
Escherichia coli numbering.
The P1-16S2 pair was used for species identification; the 16S1-16S2 pair was used for probe synthesis.
MLST.
MLST was performed as described by Homan et al. (30) and included the genes purK, adk, atpA, ddl, gdh, gyd, and pstS. Sequence types (ST) were obtained from the MLST database at http://www.mlst.net. PCR was performed in a Gene Amp PCR system 2400 thermal cycler (Applied Biosystems, Foster City, CA). Amplification reactions were carried out in a 50-μl final volume containing 2.5 U AmpliTaq Gold (Applied Biosystems). Amplified fragments were purified from the reaction mix using a Montage PCR purification kit (Millipore Corporation, Bedford, MA). Sequencing was performed using ABI Prism (Applied Biosystems) with dye-labeled terminators; sequences were analyzed by the ClustalW method available at http://align.genome.jp
Detection of glycopeptide resistance and virulence genes.
Total DNA extraction was done as described previously (3). Vancomycin resistance and virulence genes were detected by PCR using a Hybaid PCR Express thermal cycler (Hybaid Ltd, Ashford, United Kingdom). Primers and target genes are listed in Table 3. Virulence genes were detected using primers internal to (i) highly conserved regions in the AS genes of pAD1, pPD1, and pCF10 of E. faecalis, (ii) asa373 of pAM373, (iii) gelE, (iv) cylB, and (v) esp. EcoRI digestion of AGG amplicons was performed according to the manufacturer's instructions (Roche Molecular Biochemicals, Mannheim, Germany), and restriction fragments were separated by 2.0% agarose gel electrophoresis.
vanA gene location.
The plasmid or chromosomal location of vanA was assessed using three different methods: (i) vanA hybridization of plasmid content extracted by an alkaline lysis method; (ii) vanA hybridization of S1-digested total DNA; (iii) and vanA hybridization of I-CeuI-digested total DNA. The first two methods were performed as described previously (23) using a biotin-labeled vanA probe and a BrightStar BioDetect kit (Ambion, Huntingdon, United Kingdom). vanA hybridization of I-CeuI-digested total DNA was performed essentially as described previously (33). DNA was digested with 5 U of I-CeuI (New England Biolabs), separated by PFGE, transferred onto a nylon membrane, and hybridized sequentially with 16S rRNA gene and vanA biotin-labeled DNA probes.
Molecular analysis of Tn1546-like elements.
The structure of Tn1546-like elements was analyzed by PCR and amplicon restriction analysis (PCR- restriction fragment length polymorphism) essentially as described by Palepou et al. (43). Long PCR was performed using TaKaRa Ex Taq (Cambrex Bio Science, Milan, Italy) and a Hybaid PCR Express thermal cycler. Primers (sequence and position) and target genes are listed in Table 3. Long PCR amplicons of the whole Tn1546 were analyzed by digestion with ClaI, whereas amplicons of the orf2-vanX region were digested with DdeI to detect the point mutation at position 8234 (31). Strains giving different results from the prototype were amplified using primer pairs targeting the left (orf1-orf2) and right (vanX-vanZ) region of Tn1546.
Phenotypic assays.
Clumping assays were performed as described previously (40). Production of Gel was determined as described previously (11). For β-hemolysis detection, strains were grown on horse blood agar plates for 1 to 2 days at 37°C. Biofilm formation was tested using the slime production assay described previously (17).
Statistical analysis.
The prevalence of the different species, different Tn1546 types, and virulence traits in the various reservoirs were compared using Fisher's test. Statistical analysis was performed with the S-PLUS 6 statistical program (S-PLUS 6.1 for Windows, Professional Edition, release 1). A P of <0.05 was regarded as statistically significant.
RESULTS
Species and glycopeptide resistance genotype identification.
Of the 154 GRE, there were 120 E. faecium, 18 E. durans, 12 E. faecalis, and 4 E. gallinarum. Species prevalence in the different reservoirs is reported in Table 4. As expected, E. faecium was always the prevalent species (P < 0.02), followed by E. faecalis (among human specimens) and by E. durans (among nonhuman samples). E. faecalis was not found among animal nor E. durans among human samples. E. faecalis was more frequently recovered from the clinical than the other reservoirs (P < 0.05). Among the other reservoirs (i.e., animal, food, and human intestinal), the sole significant difference in its frequency was between food and animal samples (P = 0.03).
TABLE 4.
Species distribution in the different reservoirs
| Origin | No. of isolates
|
|||
|---|---|---|---|---|
| E. faecium | E. faecalis | E. durans | E. gallinarum | |
| Human intestinal | 47 | 1 | 0 | 0 |
| Animal | 35 | 0 | 12 | 2 |
| Food | 24 | 4 | 6 | 2 |
| Human clinical | 14 | 7 | 0 | 0 |
The results of multiplex PCR targeting vanA, vanB, and vanC showed that all isolates carried the vanA gene, including the four vanC-1 E. gallinarum.
PFGE typing.
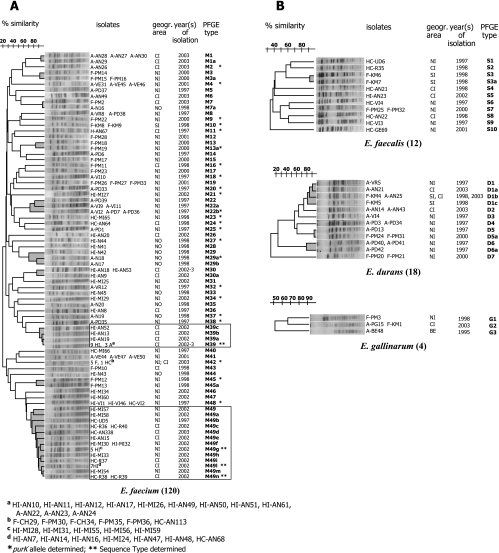
All isolates were PFGE typed after SmaI digestion of total DNA, yielding 69 different PFGE types (E. faecium M1 to M49, E. faecalis S1 to S10, E. durans D1 to D7, and E. gallinarum G1 to G3) and 30 PFGE subtypes. Results are represented in four dendrograms, one per species (Fig. 1).
FIG. 1.
Dendrograms showing the similarity index among the 154 isolates of E. faecium (A) and E. faecalis, E. durans, and E. gallinarum (B). Clusters sharing ≥70% similarity are shown in gray. A, animal isolate; F, food isolate; HC, human clinical isolate; HI, human intestinal isolate. CI, central Italy; NI, northern Italy; SI, southern Italy; NO, Norway; BE, Belgium. PFGE types showing a clonal spread are boxed.
Ten clusters of E. faecium, one of E. faecalis, and three of E. durans were evidenced. Two E. faecium PFGE types (M39 and M49) provided evidence of clonal spread; type M39 (n = 15) was isolated from both human (n = 12, in- and outpatients) and animal (n = 3, pig) samples, whereas type M49 (n = 26) was isolated from inpatients (both fecal [n = 19] and clinical [n = 7] samples). Six additional PFGE types were recovered from different sources: M7, D1, D5, and G2 from both animals and food, M29 from both human intestine and animals, and M42 from both human clinical and food (meat and cheese) samples. Type M30 was detected in fecal samples from both in- and outpatients.
With regard to geographic spread, type M7 was recovered from two isolates, one from Italy (poultry meat) and one from Norway (chicken feces), type D1 was collected throughout Italy, type D5 in northern Italy, types M39, G2, and M30 in central Italy, type M29 in Norway, and types M49 and M42 in central and northern Italy.
purK allele analysis.
purK allele polymorphisms were determined in 28 isolates of different origins (5 clinical, 6 human intestinal, 11 animal, and 6 food) and PFGE types. Five purK alleles were found, with types 1, 6, and 3 being detected in multiple strains (Table 5). Type 1 was found mostly in human strains (3/5 clinical and 5/6 intestinal), type 6 in poultry (6/10 animal and 4/5 meat), and type 3 in poultry (3/10 animal).
TABLE 5.
purK allele in 28 strains of different origins and PFGE types
| Origin and strain | Source | PFGE type | purK allele |
|---|---|---|---|
| Human intestinal | |||
| HI-VI1 | Inpatient | M48 | 1 |
| HI-MI28 | Inpatient | M49g | 1 |
| HI-MI29 | Inpatient | M34 | 1 |
| HI-AN47 | Inpatient | M49l | 1 |
| HI-AN10 | Community | M39 | 1 |
| HI-MI27 | Community | M21 | 6 |
| HI-N44 | Community | M27 | 6 |
| Animal | |||
| A-PD1 | Poultry | M25 | 2 |
| A-VI2 | Poultry | M22b | 3 |
| A-VI10 | Poultry | M18 | 3 |
| A-VR12 | Poultry | M32 | 3 |
| A-N18 | Poultry | M29a | 6 |
| A-N19 | Poultry | M37 | 6 |
| A-AN26 | Poultry | M2 | 6 |
| A-VE31 | Poultry | M4 | 6 |
| A-PD33 | Poultry | M20 | 6 |
| A-PD35 | Poultry | M38 | 6 |
| A-AN23 | Pig | M39 | 1 |
| Food | |||
| F-PM11 | Poultry | M16 | 3 |
| F-PM12 | Poultry | M45 | 6 |
| F-PM19 | Poultry | M13a | 6 |
| F-PM22 | Poultry | M9 | 6 |
| F-PM30 | Poultry | M42 | 6 |
| F-KM9 | Pork | M10 | 9 |
| Human clinical | |||
| HC-AN64 | Blood | M24 | 1 |
| HC-BG65 | Blood | M23 | 1 |
| HC-R38 | Urine | M49n | 1 |
| HC-AN67 | Urine | M11 | 3 |
MLST.
To gain a better understanding of the clonal lineage of the two major PFGE clusters, M39 and M49, MLST was performed on five isolates representing different reservoirs, two from cluster M39 (HI-AN10 and A-AN23) and three from M49 (HI-MI28, HI-AN47, and HC-R38, subtype M49g, M49l, and M49n, respectively). ST 18 was found in the two M39 isolates (one from outpatient feces and one from a pig), and ST 78 was found in the three M49 isolates (two from inpatient feces and one clinical).
One hundred and five isolates (9 E. faecalis, 78 E. faecium, 14 E. durans, and 4 E. gallinarum strains), encompassing different origins and PFGE types, were selected for further studies, i.e., vanA gene location, molecular analysis of Tn1546 elements, and virulence traits.
vanA gene location.
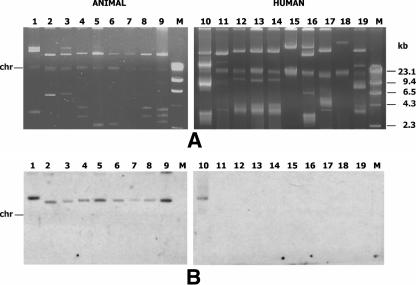
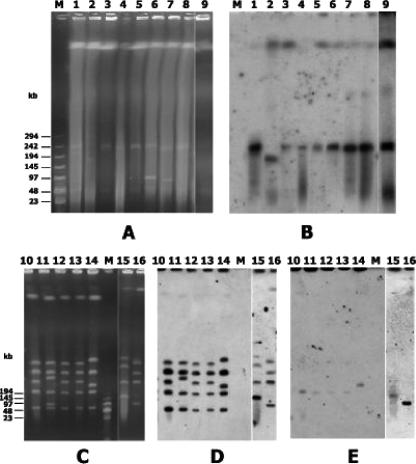
Hybridization of plasmid content following alkaline lysis extraction demonstrated a plasmid location of vanA in 77 of the 105 strains (Fig. 2). Fifty-four isolates, including the 28 that did not hybridize and 26 isolates showing a positive reaction, were subjected to vanA hybridization of S1-digested total DNA, which allows better identification of high-molecular-weight plasmids. A plasmid location of vanA was demonstrated with this method in 24 of the previously negative 28 isolates and was confirmed in all of the 26 positive ones. The size of the vanA-carrying plasmids ranged from 150 kb to 250 kb in the former and from 25 kb to 150 kb in the latter isolates. The same 54 isolates were then analyzed by sequential hybridization of I-CeuI-digested total DNA with 16S rRNA gene and vanA probes. All tested strains hybridized with the 16S rRNA gene probe, and seven strains (E. faecium HI-MI30, HI-MI25, HI-MI31, HI-MI32, and HI-MI60 and E. gallinarum A-BE48 and F-PM3) also hybridized with the vanA probe, demonstrating a chromosomal location of the vanA gene. In S1 digestion experiments, none of the E. gallinarum isolates hybridized with the vanA probe, whereas in all of the five E. faecium isolates, vanA was detected on a 240-kb plasmid, thus demonstrating the presence of two copies of vanA (Fig. 3). In E. faecium HI-AN20 and HI-AN15, the vanA location could not be assessed with any experimental approach.
FIG. 2.
Plasmid profile (A) and vanA hybridization (B) of animal (lines 1 to 9) and human (lines 10 to 19) isolates. Line 1, E. durans A-VI4; line 2, E. faecium A-PD33; line 3, E. durans A-VR5; line 4, E. faecium A-PD6; line 5, E. faecium A-VR8; line 6, E. faecium A-PD37; line 7, E. faecium A-VI9; line 8, E. faecium A-VI10; line 9, E. faecium A-VR12; line 10, E. faecium HI-MI29; line 11, E. faecium HI-MI30; line 12, E. faecium HI-MI57; line 13, E. faecium HI-MI58; line 14, E. faecium HI-MI31; line 15, E. faecium HI-MI32; line 16, E. faecium HI-MI27; line 17, E. faecium HI-MI60; line 18, E. faecium HI-MI34; line 19, E. faecium HI-MI28. M, molecular size marker (Marker II; Roche).
FIG. 3.
PFGE of S1-digested (A) and I-CeuI-digested (C) total DNA and corresponding vanA (B and E) and 16S rRNA gene (D) hybridization. Lane 1, E. faecium HI-MI28; lane 2, E. faecium HI-MI34; lane 3, E. faecium HI-MI60; lane 4, E. faecium HI-MI32; lane 5, E. faecium HI-MI31; lane 6, E. faecalis HI-MI58; lane 7, E. faecium HI-MI57; lane 8, E. faecium HI-MI30; lane 9, E. faecium HI-MI25; lane 10, E. faecium HI-MI30; lane 11, E. faecium HI-MI25; lane 12, E. faecium HI-MI31; lane 13, E. faecium HI-MI32; lane 14, E. faecium HI-MI60; lane 15, E. gallinarum A-BE48; lane 16, E. gallinarum F-PM3. M, low range marker (BioLabs).
Molecular analysis of Tn1546-like elements.
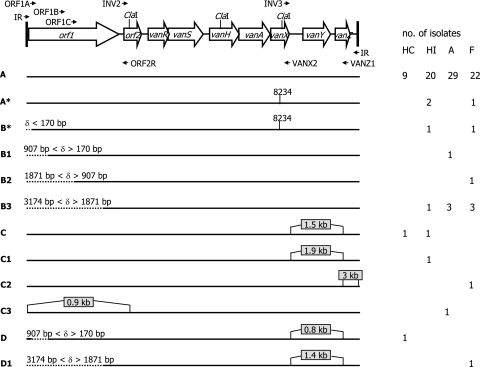
The 105 vanA isolates were analyzed for the structure of the Tn1546 element and assigned to 12 different groups (Fig. 4). Overall, PCR experiments with primer IR gave a positive result in 93 isolates. In 83 isolates, they yielded a single amplicon identical in size to the prototype Tn1546 element, as also confirmed by ClaI restriction analysis; in six isolates (HI-VI1, HI-MI29, HC-AN64, HC-VI2, F-KM5, and A-PD3), they gave rise to an amplicon larger than the prototype; and in the remaining four isolates (HI-MI25, HI-MI31, HI-MI32, and HI-MI60), they yielded two amplicons, one of the same size as the prototype and one larger.
FIG. 4.
Schematic representation of the Tn1546 prototype (A) and 11 different Tn1546-like elements (A* to D1) detected in the 101 vanA isolates carrying a single vanA element. Locations of primers, ClaI target sites, and the mutation at position 8234 are indicated. Left-side deletions (δ, deletion size) are indicated by dotted lines, insertions by gray boxes. The origin (HC, HI, A, and F) and number of isolates carrying the Tn1546 type are reported on the right. The labels of the Tn1546-like elements of Palepou et al. (43) that may correspond to those characterized in the present study are reported in parentheses: A, A* (A); B* (D); B1 (D/M); B2 (M); B3 (P); C, C1 (B/C); C2 (no correspondence); C3 (H-L); D (E); and D1 (Q-S).
The 10 isolates differing from the prototype and the 12 isolates not yielding amplicons were further studied by PCR. In the six isolates harboring a single Tn1546-like element larger than the prototype, the ORF1A-ORF2R primer pair yielded an amplicon corresponding to the prototype in four cases (E. durans F-KM5, E. faecium HI-VI1, E. faecium HC-VI2, and E. faecium HI-MI29), an amplicon larger by 900 bp in one (E. durans A-PD3), and no amplicon in the remaining isolate (E. faecium HC-AN64); the INV3-VANZ1 pair yielded an amplicon of the expected size in E. durans A-PD3 and E. durans F-KM5 (suggesting an insertion downstream of VANZ1 in this strain) and an amplicon larger by 800 bp to 1,900 bp in the remaining isolates (Fig. 4). The four isolates with two IR amplicons, one corresponding to and one larger than the prototype, all gave the same results, i.e., ClaI digestion yielded three extra fragments, suggesting the presence of a Tn1546 element 3 kb larger than the prototype. Tn1546 amplification using ORF1A-ORF2R and INV3-VANZ1, targeting the ends of the transposon, and further PCR experiments using INV2-VANX1, targeting the core region, yielded amplicons comparable in size to those of the prototype, indicating the presence of insertions downstream of VANZ1 or upstream of ORF1A (data not shown).
Primer pair INV3-VANZ1 yielded an amplicon corresponding to the prototype in all 12 isolates giving no IR amplicons, indicating a prototype vanA cluster on the right end, whereas ORF1A-ORF2R yielded an amplicon of the expected size in only two isolates (E. faecium F-KM8 and HI-AN18), arguing for the presence in these strains of deletions upstream of nucleotide 170 (Fig. 4). Additional PCR experiments were performed to establish the size of the left-end deletion in the remaining 10 isolates. Primer pair ORF1B-ORF2R yielded a product corresponding to the prototype in one strain (E. faecium A-AN26), suggesting a left-side deletion until a nucleotide between 170 and 907 bp, while ORF1C-ORF2R yielded a product corresponding to the prototype in one of the remaining strains (E. faecalis F-KM6), suggesting a left-end Tn1546 deletion as far as a nucleotide between 907 and 1871. No amplicons were obtained from the remaining eight isolates, arguing for an orf1 deletion until a nucleotide between 1871 and 3174.
Tn1546 elements were also analyzed for the presence of the G-to-T mutation at position 8234 in the vanX gene using INV2-VANX1 and DdeI digestion of amplicons. Restriction analysis revealed the vanX mutation in five E. faecium isolates, three human intestinal (HI-AN9, HI-AN18, and HI-MI34) and two pork (F-KM8 and F-KM9) isolates (Fig. 4).
Fisher's test failed to evidence any association between Tn1546 type and a particular source of GRE or a particular enterococcal species (P > 0.5).
Genetic detection and expression of virulence determinants.
The 105 vanA enterococcal isolates were screened for the presence of AS genes, gelE, cylB, and esp and tested for clumping after growth in the presence of pheromone-containing supernatants of E. faecalis JH2-2 and E. faecalis OG1RF and for gelatinase and hemolysin production.
The presence of AS genes was determined by PCR using primers AGG and ASA373. To identify the specific AS gene, AGG amplicons were subjected to EcoRI restriction analysis, together with those obtained with E. faecalis OG1RF(pCF10, prgB) and E. faecalis OG1RF(pAD1, asa1) (Fig. 5). All tested E. faecalis isolates carried at least one AS gene. In particular, HI-AN23, HC-N22, and F-PM25 carried prgB, whereas HC-VI4 was shown to contain both prgB and asa1 genes, as well as asa373. Four strains showed a restriction profile with an additional fragment, and the corresponding AS genes were indicated as prgB* (HC-UD6, F-KM6, and F-KM7) and asa1* (HC-R35). HC-AN21 contained both prgB* and asa1.
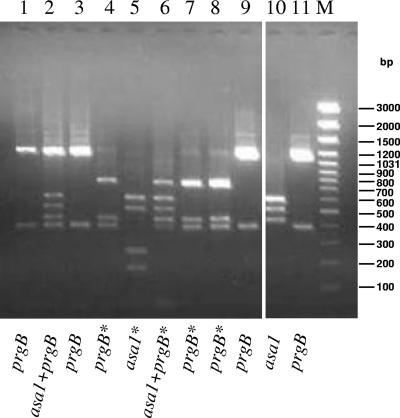
FIG. 5.
EcoRI restriction analysis of AGG amplicons of nine E. faecalis isolates and of E. faecalis OG1RF(pAD1, asa1) and E. faecalis OG1RF(pCF10, prgB) reference strains. Lane 1, HI-AN23; line 2, HC-VI4 (also asa373 positive); line 3, HC-AN22; line 4, HC-UD6; line 5, HC-R35; line 6, HC-AN21; line 7, F-KM6; line 8, F-KM7; line 9, F-PM25; line 10, OG1RF(pAD1; asa1); and line 11, OG1RF(pCF10; prgB). *, additional EcoRI site. M, GeneRuler 100-bp DNA Ladder Plus marker (M-Medical Genenco).
A total of 21 vanA isolates exhibited a positive clumping reaction. Growth in the presence of pheromones gave rise to different levels of aggregation: some strains generated moderate to large aggregates, whereas others elicited a barely detectable effect (Table 6). Clumps were particularly evident in six (five human and one food) E. faecalis isolates, all containing one or more of the AS genes tested (HC-VI4 [prgB, asa1, and asa373], HC-AN21 [prgB* and asa1], HC-AN22 [prgB], HC-UD6 [prgB*], HI-AN23 [prgB], and F-PM25 [prgB]). Clumps were less pronounced in the remaining 15 isolates (8 human, 5 animal, and 2 food; 12 E. faecium, 2 E. durans and 1 E. gallinarum), none of which contained any of the AS genes tested. The clumping-negative phenotype correlated with the presence of an additional EcoRI site in E. faecalis HC-R35 (asa1*), F-KM6 (prgB*), and F-KM7 (prgB*).
TABLE 6.
PFGE and Tn1546 type, vanA location, genotype or phenotype of virulence, and slime production of 48 vanA enterococcal isolates of different origins harboring one or more virulence traitsa
| Origin (n) and strain | Species | PFGE type | vanA location | Tn1546 type | Genotype or phenotype of virulence
|
Slime production | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gelE | Gel | esp | AS genes | Clumping activity | ||||||||||||||||
| Human intestinal (13) | ||||||||||||||||||||
| HI-AN19 | E. faecium | M39a | P* | A | + | − | − | − | − | − | ||||||||||
| HI-MI32 | E. faecium | M49f | P* + Chr | A/C2 | + | − | − | − | − | − | ||||||||||
| HI-MI57 | E. faecium | M49 | P* | A | + | − | − | − | − | − | ||||||||||
| HI-VI1 | E. faecium | M48 | P | C | + | − | + | − | − | − | ||||||||||
| HI-AN8 | E. faecium | M36 | P | B3 | − | − | − | − | + | − | ||||||||||
| HI-AN15 | E. faecium | M49e | ND | A | − | − | − | − | + | − | ||||||||||
| HI-MI30 | E. faecium | M49f | P* + Chr | A | + | − | − | − | + | − | ||||||||||
| HI-MI54 | E. faecium | M49m | P* | A | + | − | − | − | + | − | ||||||||||
| HI-MI58 | E. faecium | M49a | P* | A | − | − | − | − | − | WP | ||||||||||
| HI-N42 | E. faecium | M29 | P | A | − | − | − | − | + | − | ||||||||||
| HI-N43 | E. faecium | M44 | P | A | − | − | − | − | + | − | ||||||||||
| HI-N44 | E. faecium | M27 | P | A | − | − | − | − | + | − | ||||||||||
| HI-AN23 | E. faecalis | S5 | P | A | − | − | − | prgB | ++ | SP | ||||||||||
| Animal (15) | ||||||||||||||||||||
| A-VE44 | E. faecium | M41 | P* | B3 | − | − | − | − | − | WP | ||||||||||
| A-VI2 | E. faecium | M22b | P | A | + | − | − | − | − | − | ||||||||||
| A-PD1 | E. faecium | M25 | P | A | + | − | − | − | − | − | ||||||||||
| A-PD6 | E. faecium | M14 | P | A | + | − | − | − | − | − | ||||||||||
| A-PD35 | E. faecium | M38 | P | A | + | − | − | − | − | − | ||||||||||
| A-VI19 | E. faecium | M22a | P | A | + | − | − | − | − | − | ||||||||||
| A-VI10 | E. faecium | M18 | P | A | + | − | − | − | − | − | ||||||||||
| A-VR12 | E. faecium | M32 | P | A | + | − | − | − | − | − | ||||||||||
| A-N19 | E. faecium | M37 | P | A | − | − | − | − | + | − | ||||||||||
| A-N20 | E. faecium | M35 | P | B3 | − | − | − | − | + | − | ||||||||||
| A-VR8 | E. faecium | M8 | P | A | − | − | − | − | + | − | ||||||||||
| A-AN21 | E. durans | D1a | P | A | − | − | − | − | − | SP | ||||||||||
| A-AN14 | E. durans | D2 | P | A | − | − | − | − | + | − | ||||||||||
| A-PD41 | E. durans | D6 | P | A | − | − | − | − | + | − | ||||||||||
| A-PG15 | E. gallinarum | G1 | P | A | + | − | − | − | − | − | ||||||||||
| Food (11) | ||||||||||||||||||||
| F-KM9 | E. faecium | M10 | P | A* | + | − | − | − | − | − | ||||||||||
| F-PM12 | E. faecium | M45 | P | A | + | − | − | − | − | − | ||||||||||
| F-PM15 | E. faecium | M3a | P | A | + | − | − | − | − | − | ||||||||||
| F-PM13 | E. faecium | M45a | P | A | − | − | − | − | + | − | ||||||||||
| F-KM6 | E. faecalis | S3 | P | B2 | + | + | − | prgB* | − | SP | ||||||||||
| F-KM7 | E. faecalis | S3a | P | A | + | + | − | prgB* | − | WP | ||||||||||
| F-PM25 | E. faecalis | S7 | P | A | + | + | − | prgB | +++ | SP | ||||||||||
| F-KM5 | E. durans | D1c | P | C2 | + | − | − | − | − | − | ||||||||||
| F-PM20 | E. durans | D7 | P | B3 | + | − | − | − | − | − | ||||||||||
| F-PM3 | E. gallinarum | G1 | Chr | D1 | − | − | − | − | + | − | ||||||||||
| F-KM1 | E. gallinarum | G2 | P | A | − | − | + | − | − | − | ||||||||||
| Human clinical (9) | ||||||||||||||||||||
| HC-AN64 | E. faecium | M24 | P | D | − | − | + | − | − | − | ||||||||||
| HC-UD5 | E. faecium | M49b | P | A | − | − | + | − | − | − | ||||||||||
| HC-VI2 | E. faecium | M48 | P | C | − | − | + | − | + | − | ||||||||||
| HC-R38 | E. faecium | M49n | P* | A | + | − | − | − | − | − | ||||||||||
| HC-VI4 | E. faecalis | S6 | P | A | + | + | − | prgB asa1 asa373 | +++ | − | ||||||||||
| HC-AN21 | E. faecalis | S4 | P | A | + | + | − | prgB* asa1 | ++ | SP | ||||||||||
| HC-AN22 | E. faecalis | S8 | P | A | + | + | − | prgB | ++ | WP | ||||||||||
| HC-UD6 | E. faecalis | S1 | P | A | + | + | − | prgB* | ++ | − | ||||||||||
| HC-R35 | E. faecalis | S2 | P | A | + | + | − | asa1* | − | SP | ||||||||||
Abbreviations: P, <150-kb plasmid; P*, >150-kb plasmid; Chr, chromosome; Gel, gelatinase production; WP, weak producer (0.120 < optical density < 0.240); SP, strong producer (optical density > 0.240); ND, not detected.
gelE was detected in 28 isolates, of which eight (five human and three food) were also Gel producers (Table 6). All the gelE-positive Gel producers were E. faecalis isolates and carried at least one AS gene (F-KM6, F-KM7, F-PM25, HC-VI4, HC-AN21, HC-AN22, HC-UD6, and HC-R35). No Gel production was detected in the remaining gelE-positive strains (17 E. faecium [A-PD1, A-VC2, A-PD35, A-PD6, A-VI9, A-VI10, A-VR12, F-KM9, F-PM12, F-PM15, HC-R38, HI-VI1, HI-AN19, HI-MI54, HI-MI30, HI-MI57, and HI-MI32], 2 E. durans [F-KM5 and F-PM20], and 1 E. gallinarum [A-PG15]). The five esp-positive strains (Table 6) included four E. faecium isolates (HC-AN64, HC-VI2, HC-UD5, and HI-VI1) and an E. gallinarum isolate (F-KM1). cylB was not detected in any of the 105 enterococcal isolates, all of which were negative for β-hemolysis.
Biofilm formation.
When the 105 vanA isolates were tested in vitro for biofilm formation on abiotic surfaces, 10 strains were seen to have a strong or weak ability to produce biofilm (Table 6). Seven of these strains were E. faecalis (four human and three food), two E. faecium (one human and one animal), and one E. durans (animal). None of the four E. gallinarum strains were able to form biofilm. Interestingly, the seven positive E. faecalis strains (five strong and two weak producers) were also positive for prgB and/or asa1 and gelE and were negative for esp. These features were independent of the source of isolation. In contrast, the two E. faecium weak biofilm producers and the only E. durans isolate (a strong biofilm producer) were negative for all tested virulence traits (Table 6).
Overall, strains possessing suspected virulence genes were more frequent among clinical isolates than in the other reservoirs (P < 0.05), whereas there was no significant difference in their occurrence between human intestinal, animal, and food isolates (P > 0.6). However, the occurrence of strains carrying multiple virulence factors was peculiar to clinical and food reservoirs only.
DISCUSSION
Since the 1997 European Union ban forbidding the use of avoparcin in animal feeds, the prevalence of GRE has decreased among farm animals and in the community (15, 34, 54), even though a readily detectable persistence of GRE in avoparcin-exposed farm environments has been reported (1, 33). By contrast, the incidence of GRE in hospitals has remained substantially unchanged in northern Europe and has actually increased in southern European countries (http://www.earss.rivm.nl). The role of different reservoirs in the spread of glycopeptide resistance is thus still unclear.
In this study, we compared GRE isolates of different origins and geographic locations. GRE were initially identified at the species level, PFGE typed, and analyzed for their van genotypes. Results showed different species prevailing in the different reservoirs. As expected, E. faecium was the most prevalent species irrespective of the source, whereas E. faecalis was recovered only from human (mostly clinical) and food samples. E. durans and E. gallinarum were only isolated from animal and food samples. All isolates were confirmed to be vanA positive and vanB negative.
PFGE results showed a polyclonal distribution of vanA isolates in the different reservoirs; however, the presence of some clones in different reservoirs was observed. In particular, 26 E. faecium isolates belonged to type M49; they were first isolated in northern Italy in 1997 and since 2002 in northern and central Italy, thus showing both a temporal and a geographic spread. A different subtype characterized the different hospitals or towns of isolation. The same clone was isolated from both clinical and intestinal human samples, suggesting an ability of intestinal isolates to act as pathogens. However, no difference in virulence determinants was detected between clinical and intestinal clonally related E. faecium isolates. Clinical isolates could act as opportunistic pathogens or could have acquired virulence traits still to be characterized. Notably, the M49 type and subtypes belong to ST 78, which has already been described as epidemic in Italy (6). Type M39 was recovered over a limited period of time (2002 to 2003) and geographic area (central Italy) from human and pig intestines, suggesting an ability to colonize both species. It was shown to belong to ST 18, which has never been found in either human epidemic or animal GRE according to a previous Italian study (6). Interestingly, ST 18 and ST 78 both belong to the clonal complex 17 (CC17), the first globally dispersed nosocomial-adapted clonal lineage of E. faecium (56). However, while type M49 (ST 78) was isolated from intestinal samples of inpatients only, type M39 (ST 18) was isolated from intestinal samples of inpatients as well as in the community and in pigs. Isolates belonging to types M7, M29, M42, D1, D5, and G2 were also recovered from samples of different origins, demonstrating an occasional clonal spread between different reservoirs also for E. durans and E. gallinarum. M29 and M42, as well as other PFGE types isolated from poultry in different geographic areas, carried the purK type 6 allele, whereas all human hospital isolates (except type M11) carried purK type 1, according to data reported by others (6, 33, 50). Overall, these data strongly suggest that human colonization by food and animal GRE is possible but that vertical transmission between different reservoirs is infrequent. Although the colonization might be transient (49, 57), the possible transfer of resistance genes during this period could be crucial.
Results of Tn1546 location analyses suggested an association among the vanA location, species, and origin of isolates. In the vast majority of our strains (98%), vanA was located on plasmids of either <150 kb (all the E. faecalis and E. durans strains) or >150 kb (intestinal E. faecium, both human—including M49 and M39—and animal). Notably, plasmids of >150 kb have already been described in an E. faecium clone widely disseminated among pigs (1). These results suggest that high-molecular-weight E. faecium plasmids might be involved in intestinal colonization of both humans and animals, thus contributing to the persistence of resistant strains. Moreover, large plasmids are likely to be conjugative, thus contributing to the horizontal transfer processes. A chromosomal vanA location was demonstrated in two of the four E. gallinarum strains, suggesting that in this species the chromosome is a more common location than in other enterococcal species, as also reported previously (23).
Tn1546 typing showed a Tn1546 element, indistinguishable from the prototype, in about 80% of the strains tested. The remaining Tn1546-like elements displayed insertions or left-end deletions. This finding agrees with other data from Italian vanA strains from different sources (5). In contrast, Tn1546-like elements different from the prototype seem to be more common in other countries (8, 26, 32, 46, 57). The same Tn1546 type was found in clonally unrelated poultry, swine, and human strains (Fig. 4), while different Tn1546 types were found in isolates belonging to the same clone (Table 6), suggesting that horizontal gene transfer may have played a significant role in the spread of glycopeptide-resistant strains. The finding of the G-to-T mutation at position 8234 of Tn1546 in pork and human isolates, suggesting a relationship between human and food vanA elements, supports this hypothesis.
When evaluated for virulence determinants and their expression, about half of the GRE showed at least one virulence trait, gelE and the pheromone response being the most frequent. Gelatinase production was found in all clinical and food E. faecalis isolates. Since gelatinase production has been more frequently described in clinical isolates than in those from other sources (11, 13), these results point to a link between clinical and food reservoirs, as suggested by previous reports (9, 47). Silent gelE was detected in isolates from other species (E. faecium [particularly human and animal feces], E. durans [food], and also E. gallinarum [animal feces]). gelE has been documented frequently in E. faecalis, rarely in E. faecium and E. durans (20, 24), but never in E. gallinarum. Thus, the spread of gelE from E. faecalis by horizontal gene transfer might be involved in the evolution of different pathogenic enterococcal species. Lack of expression in species other than E. faecalis might be explained by low levels or downregulation of gene expression, an inactive gene product, or experimental conditions. Growth in the presence of E. faecalis sex pheromones gave rise to clumps in all species, although the level of aggregation was higher in E. faecalis, suggesting a species-specific response. On the other hand, AS genes (prevalently prgB) were detected only in E. faecalis, with some strains harboring more than one gene (prgB, asa1, and asa373). AS genes were also detected in a few clumping-negative isolates. The presence in some strains of prgB* or asa1* correlated with the clumping-negative phenotype, suggesting an inactive gene product.
Although it is frequently carried by pheromone-response plasmids (24, 27), cylB was never detected; esp was only detected in a few E. faecium (human) and E. gallinarum (food) isolates, arguing against the association of these virulence traits with glycopeptide resistance. Although the ability to form biofilm was uncommon, it is worth noting that it was mostly present in vanA E. faecalis isolates harboring other virulence genes.
Overall, virulence studies of our vanA enterococci revealed different trends in the occurrence of virulence determinants among human, food, and animal isolates. Their higher incidence detected among human and food compared with animal isolates was associated with the presence of E. faecalis isolates carrying multiple virulence factors in the former reservoirs.
A similar virulence profile observed among clinical and food E. faecalis isolates and the absence of multivirulent animal enterococci suggest that food could be more closely involved than animals in the spread of virulent GRE in humans. Moreover, the different features observed in enterococcal strains isolated from breeding animals and animal food raise questions about the source of food contamination by vanA enterococci. On the other hand, GRE polyclonality suggests that horizontal transfer of the vanA cluster, rather than clonal spread, is responsible for their emergence and dissemination. Subsequently, the presence of vanA, combined with one or more virulence genes in the same genome, could have favored particular clusters of E. faecium, e.g., type M49 in the hospital environment.
The E. faecium isolates were generally devoid of virulence determinants, albeit with notable exceptions. Although all E. faecium strains lacked the AGG genes, several strains formed clumps after pheromone induction. The same profile was occasionally observed in E. durans and E. gallinarum isolates. This phenomenon might depend on the presence of AS/pheromone systems in these species, different from those of E. faecalis. By contrast, using E. faecalis primers, gelE and esp determinants were detected in several E. faecium and in some E. durans and E. gallinarum isolates, in line with data on E. faecium reported by Eaton et al. (20, 21). The presence in the different reservoirs of vanA E. faecium, E. durans, and E. gallinarum isolates carrying virulence factors might be linked with the increasing isolation rate of enterococcal species other than E. faecalis in hospital settings (4).
In conclusion, (i) indistinguishable plasmid-located vanA determinants in PFGE-diverse populations strongly suggest a common Tn1546 reservoir readily accessible by horizontal gene transfer; (ii) large vanA plasmids (>150 kb) carried by intestinal E. faecium might be involved in GRE colonization/infection in humans (with colinked unknown virulence determinants possibly involved in adhesion) and represent an important target for further studies; and (iii) detection of the same vanA and virulence determinants in enterococci of different species and origins, in the presence of host-specific purK housekeeping genes, indicates a lack of host-specific markers and suggests that all GRE, irrespective of their origins and species, might be regarded as potential reservoirs of resistance determinants and virulence traits transferable to human-adapted clusters. Moreover, the finding of virulent vanA E. faecalis in meat suggests a food involvement (farm animal independent) in the spread of virulent GRE in humans.
Acknowledgments
We are grateful to Gary Dunny for supplying E. faecalis strains OG1RF and OG1RF(pCF10) and to Pier Sandro Cocconcelli for OG1RF(pAD1). Thanks also to Annalisa Cavallero (Ospedale S. Raffaele, Milano, Italy), Esther Manso (Azienda Ospedaliera Umberto I, Ancona, Italy), Giuseppina Scagnelli (Department of Infectious Diseases, San Bortolo Hospital, Vicenza, Italy), and Roberta di Rosa (Department of Clinical Medicine, University La Sapienza, Roma, Italy) for providing human GRE; to Annalisa Pantosti (Istituto Superiore di Sanità, Roma, Italy) and Francesca Clementi (Polytechnic University of Marche, Ancona, Italy) for providing animal and food strains; and to Roberta Fontana (University of Verona, Italy) and Anna Grossato (University of Padova, Italy) for providing animal strains. We are also grateful to Luigi Ferrante for his assistance with statistical analyses and to Manuela Vecchi for sequencing experiments.
This study was supported by the European research project “Antimicrobial Resistance Transfer from and between Gram-Positive Bacteria of the Digestive Tract and Consequences for Virulence” (ARTRADI), contract QLK2-CT-2002-00843.
Footnotes
Published ahead of print on 9 March 2007.
REFERENCES
- 1.Aarestrup, F. M. 2000. Characterization of glycopeptide-resistant Enterococcus faecium (GRE) from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J. Clin. Microbiol. 38:2774-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur, M., P. E. Reynolds, F. Depardieu, S. Evers, S. Dutka Malen, R. J. Quintiliani, and P. Courvalin. 1996. Mechanisms of glycopeptide resistance in enterococci. J. Infect. 32:11-16. [DOI] [PubMed] [Google Scholar]
- 3.Biavasco, F., A. Miele, C. Vignaroli, E. Manso, R. Lupidi, and P. E. Varaldo. 1996. Genotypic characterization of a nosocomial outbreak of vanA Enterococcus faecalis. Microb. Drug Res. 2:231-237. [DOI] [PubMed] [Google Scholar]
- 4.Biavasco, F., C. Paladini, C. Vignaroli, G. Foglia, E. Manso, and P. E. Varaldo. 2001. Recovery from a single blood culture of two Enterococcus gallinarum isolates carrying both vanC-1 and vanA cluster genes and differing in glycopeptide susceptibility. Eur. J. Clin. Microbiol. Infect. Dis. 20:309-314. [DOI] [PubMed] [Google Scholar]
- 5.Bonora, M. G., C. Bordin, L. Bragagnolo, L. Girelli, M. De Fatima, A. Grossato, M. Ligozzi, G. Lo Cascio, and R. Fontana. 2001. Molecular analysis of vanA enterococci isolated from humans and animals in northeastern Italy. Microb. Drug Resist. 7:247-255. [DOI] [PubMed] [Google Scholar]
- 6.Bonora, M. G., M. Ligozzi, M. De Fatima, L. Bragagnolo, A. Goglio, G. C. Guazzotti, and R. Fontana. 2004. Vancomycin-resistant Enterococcus faecium isolates causing hospital outbreaks in northern Italy belong to the multilocus sequence typing C1 lineage. Microb. Drug Resist. 10:114-123. [DOI] [PubMed] [Google Scholar]
- 7.Bonten, M. J., R. Willems, and R. A. Weinstein. 2001. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect. Dis. 1:314-325. [DOI] [PubMed] [Google Scholar]
- 8.Brown, A. R., A. C. Townsley, and S. G. B. Amyes. 2001. Diversity of Tn1546 elements in clinical isolates of glycopeptide-resistant enterococci from Scottish hospitals. Antimicrob. Agents Chemother. 45:1309-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cetinkaya, Y., P. Falk, and C. G. Mayhall. 2000. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 13:686-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clewell, D. B., and G. M. Dunny. 2002. Conjugation and genetic exchange in enterococci, p. 265-300. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. American Society for Microbiology, Washington, DC.
- 11.Coque, T. M., J. E. Patterson, J. M. Steckelberg, and B. E. Murray. 1995. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J. Infect. Dis. 171:1223-1229. [DOI] [PubMed] [Google Scholar]
- 12.Coque, T. M., J. F. Tomayko, S. C. Ricke, P. C. Okhyusen, and B. E. Murray. 1996. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob. Agents Chemother. 40:2605-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creti, R., M. Imperi, L. Bertuccini, F. Faretti, G. Orefici, R. Di Rosa, and L. Baldassarri. 2004. Survey for virulence determinants among Enterococcus faecalis isolated from different sources. J. Med. Microbiol. 53:13-20. [DOI] [PubMed] [Google Scholar]
- 14.Dahl, K. H., E. W. Lundblad, T. P. Rokenes, O. Olsvik, and A. Sundsfjord. 2000. Genetic linkage of the vanB2 gene cluster to Tn5382 in vancomycin-resistant enterococci and characterization of two novel insertion sequences. Microbiology 146:1469-1479. [DOI] [PubMed] [Google Scholar]
- 15.Del Grosso, M., A. Caprioli, P. Chinzari, M. C. Fontana, G. Pezzati, A. Manfrin, E. D. Giannatale, E. Goffredo, and A. Pantosti. 2000. Detection and characterization of vancomycin-resistant enterococci in farm animals and raw meat products in Italy. Microb. Drug Resist. 6:313-318. [DOI] [PubMed] [Google Scholar]
- 16.Donelli, G., and E. Guaglianone. 2004. Emerging role of Enterococcus spp. in catheter-related infections: biofilm formation and novel mechanisms of antibiotic resistance. J. Vasc. Access 5:3-9. [DOI] [PubMed] [Google Scholar]
- 17.Donelli, G., C. Paoletti, L. Baldassarri, E. Guaglianone, R. Di Rosa, G. Magi, C. Spinaci, and B. Facinelli. 2004. Sex pheromone-response, clumping and slime production in enterococcal strains isolated from occluded biliary stents. J. Clin. Microbiol. 42:3419-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutka-Malen, S., S. Evers, and P. Courvalin. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton, T. J., and M. J. Gasson. 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eaton, T. J., and M. J. Gasson. 2002. A variant enterococcal surface protein Espfm in Enterococcus faecium; distribution among food, commensal, medical, and environmental isolates. FEMS Microbiol. Lett. 216:269-275. [DOI] [PubMed] [Google Scholar]
- 22.Facklam, R. R., D. F. Sahm, and L. M. Teixeira. 2005. Enterococcus, p. 297-305. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 23.Foglia, G., M. Del Grosso, C. Vignaroli, P. Bagnarelli, P. E. Varaldo, A. Pantosti, and F. Biavasco. 2003. Molecular analysis of Tn1546-like elements mediating high-level vancomycin resistance in Enterococcus gallinarum. J. Antimicrob. Chemother. 52:772-775. [DOI] [PubMed] [Google Scholar]
- 24.Gilmore, M. S., P. S. Coburn, S. R. Nallapareddy, and B. E. Murray. 2002. Enterococcal virulence, p. 301-354. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. American Society for Microbiology, Washington, DC.
- 25.Goossens, H., D. Jabes, R. Rossi, C. Lammens, G. Privitera, and P. Courvalin. 2003. European survey of vancomycin-resistant enterococci in at-risk hospital wards and in vitro susceptibility testing of ramoplanin against these isolates. J. Antimicrob. Chemother. 51(Suppl. 3):iii5-iii12. [DOI] [PubMed] [Google Scholar]
- 26.Guardabassi, L., and A. Dalsgaard. 2004. Occurrence, structure, and mobility of Tn1546-like elements in environmental isolates of vancomycin-resistant enterococci. Appl. Environ. Microbiol. 70:984-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hancock, L. E., and M. S. Gilmore. 2000. Pathogenicity of enterococci, p. 251-258. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, DC.
- 28.Heaton, M. P., L. F. Discotto, M. J. Pucci, and S. Handwerger. 1996. Mobilization of vancomycin resistance by transposon-mediated fusion of a VanA plasmid with an Enterococcus faecium sex pheromone-response plasmid. Gene 171:9-17. [DOI] [PubMed] [Google Scholar]
- 29.Hershberger, E., S. F. Oprea, S. M. Donabedian, M. Perri, P. Bozigar, P. Bartlett, and M. J. Zervos. 2005. Epidemiology of antimicrobial resistance in enterococci of animal origin. J. Antimicrob. Chemother. 55:127-130. [DOI] [PubMed] [Google Scholar]
- 30.Homan, W. L., D. Tribe, S. Poznanski, M. Li, G. Hogg, E. Spalburg, G. D. A. van Embden, and R. J. L. Willems. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen, L. B. 1998. Differences in the occurrence of two base pair variants of Tn1546 from vancomycin-resistant enterococci from humans, pigs, and poultry. Antimicrob. Agents Chemother. 42:2463-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen, L. B., P. Ahrens, L. Dons, R. N. Jones, A. M. Hammerum, and F. M. Aarestrup. 1998. Molecular analysis of Tn1546 in Enterococcus faecium isolated from animals and humans. J. Clin. Microbiol. 36:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnsen, P. J., J. I. Osterhus, H. Sletvold, M. Sorum, H. Kruse, K. Nielsen, G. S. Simonsen, and A. Sundsfjord. 2005. Persistence of animal and human glycopeptide-resistant enterococci on two Norwegian poultry farms formerly exposed to avoparcin is associated with a widespread plasmid-mediated vanA element within a polyclonal Enterococcus faecium population. Appl. Environ. Microbiol. 71:159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klare, I., D. Badstubner, C. Konstabel, G. Bohme, H. Claus, and W. Witte. 1999. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb. Drug Resist. 5:45-52. [DOI] [PubMed] [Google Scholar]
- 35.Klijn, N., A. H. Weerkamp, and W. M. de Vos. 1995. Detection and characterization of lactose-utilizing Lactococcus spp. in natural ecosystems. Appl. Environ. Microbiol. 61:788-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knijff, E., F. Dellaglio, A. Lombardi, C. Andrighetto, and S. Torriani. 2001. Rapid identification of Enterococcus durans and Enterococcus hirae by PCR with primers targeted to the ddl genes. J. Microbiol. Methods 47:35-40. [DOI] [PubMed] [Google Scholar]
- 37.Kühn, I., A. Iversen, M. Finn, C. Greko, L. Burman, A. R. Blanch, X. Vilanova, A. Manero, H. Taylor, J. Caplin, L. Domínguez, I. A. Herrero, M. A. Moreno, and R. Möllby. 2005. Occurrence and relatedness of vancomycin-resistant enterococci in animals, humans, and the environment in different European regions. Appl. Environ. Microbiol. 71:5383-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leclercq, R., E. Derlot, J. Duval, and P. Courvalin. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319:157-161. [DOI] [PubMed] [Google Scholar]
- 39.Low, D. E., N. Keller, A. Barth, and R. N. Jones. 2001. Clinical prevalence, antimicrobial susceptibility, and geographic resistance patterns of enterococci: results from the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S133-S145. [DOI] [PubMed] [Google Scholar]
- 40.Magi, G., R. Capretti, C. Paoletti, M. Pietrella, L. Ferrante, F. Biavasco, P. E. Varaldo, and B. Facinelli. 2003. Presence of a vanA-carrying pheromone response plasmid (pBRG1) in a clinical isolate of Enterococcus faecium. Antimicrob. Agents Chemother. 47:1571-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miele, A., M. Bandiera, and B. P. Goldstein. 1995. Use of primers selective for vancomycin resistance genes to determine van genotype in enterococci and to study gene organization in VanA isolates. Antimicrob. Agents Chemother. 39:1772-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nallapareddy, S. R., H. Wenxiang, G. M. Weinstock, and B. E. Murray. 2005. Molecular characterization of a widespread, pathogenic, and antibiotic resistance-receptive Enterococcus faecalis lineage and dissemination of its putative pathogenicity island. J. Bacteriol. 16:5709-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palepou, M. F., A. M. Adebiyi, C. H. Tremlett, L. B. Jensen, and N. Woodford. 1998. Molecular analysis of diverse elements mediating VanA glycopeptide resistance in enterococci. J. Antimicrob. Chemother. 42:605-612. [DOI] [PubMed] [Google Scholar]
- 44.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 45.Sahm, D. F., J. Kissinger, M. S. Gilmore, P. R. Murray, R. Mulder, J. Solliday, and B. Clarke. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schouten, M. A., R. J. L. Willems, W. A. G. Kraak, J. Top, J. A. A. Hoogkamp Korstanje, and A. Voss. 2001. Molecular analysis of Tn1546-like elements in vancomycin-resistant enterococci isolated from patients in Europe shows geographic transposon type clustering. Antimicrob. Agents Chemoter. 45:986-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semedo, T., M. A. Santos, M. F. Lopes, J. J. Figueiredo Marques, M. T. Barreto Crespo, and R. Tenreiro. 2003. Virulence factors in food, clinical and reference Enterococci: a common trait in the genus? Syst. Appl. Microbiol. 26:13-22. [DOI] [PubMed] [Google Scholar]
- 48.Shankar, N., A. S. Baghdayan, and M. Gilmore. 2002. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 417:746-750. [DOI] [PubMed] [Google Scholar]
- 49.Sorensen, T. L., M. Blom, D. L. Monnet, N. Fromodt-Moller, R. L. Poulsen, and F. Espersen. 2001. Transient intestinal carriage after ingestion of antibiotic-resistant Enterococcus faecium from chicken and pork. N. Engl. J. Med. 16:1161-1166. [DOI] [PubMed] [Google Scholar]
- 50.Sørum, M., P. J. Jhonsen, B. Aasnes, T. Rosvoll, H. Kruse, A. Sundsfjord, and G. S. Simonsen. 2006. Prevalence, persistence, and molecular characterization of glycopeptide-resistant enterococci in Norwegian poultry and poultry farmers 3 to 8 years after the ban on avoparcin. Appl. Environ. Microbiol. 72:516-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundsfjord, A., G. S. Simonsen, and P. Courvalin. 2001. Human infections caused by glycopeptide-resistant Enterococcus spp.: are they a zoonosis? Clin. Microbiol. Infect. 7(Suppl. 4):16-33. [DOI] [PubMed] [Google Scholar]
- 52.Sussmuth, S. D., A. Muscholl-Silberhorn, R. Wirth, M. Susa, R. Marre, and E. Rozdzinski. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 68:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van den Bogaard, A. E., N. Bruinsma, and E. E. Stobberingh. 2000. The effect of banning avoparcin on VRE carriage in The Netherlands. J. Antimicrob. Chemother. 46:146-147. [DOI] [PubMed] [Google Scholar]
- 55.Weaver, K. E. 2000. Enterococcal genetics, p. 259-271. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, DC.
- 56.Willems, R. J. L., J. Top, M. van Santen, D. A. Robinson, T. M. Coque, F. Baquero, H. Grundmann, and M. J. M. Bonten. 2005. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg. Infect. Dis. 11:821-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodford, N., A. M. A. Adebiyi, M. F. I. Palepou, and B. D. Cookson. 1998. Diversity of VanA glycopeptide resistance elements in enterococci from humans and nonhuman sources. Antimicrob. Agents Chemother. 42:502-508. [DOI] [PMC free article] [PubMed] [Google Scholar]