Abstract
Ionizing radiation effectively inactivates Escherichia coli O157:H7, but the efficacy of the process against biofilm cells versus that against free-living planktonic cells is not well documented. The radiation sensitivity of planktonic or biofilm cells was determined for three isolates of E. coli O157:H7 (C9490, ATCC 35150, and ATCC 43894). Biofilms were formed on sterile glass slides incubated at 37°C for either 24 h, 48 h, or 72 h. The biofilm and planktonic cultures were gamma irradiated at doses ranging from 0.0 (control) to 1.5 kGy. The dose of radiation value required to reduce the population by 90% (D10) was calculated for each isolate, culture, and maturity based on viable populations at each radiation dose. For each of the times sampled, the D10 values of isolate 43894 planktonic cells (0.454 to 0.479 kGy) were significantly (P < 0.05) higher than those observed for biofilm cells (0.381 to 0.385 kGy), indicating a significantly increased sensitivity to irradiation for cells in the biofilm habitat. At the 24-h sampling time, isolate C9490 showed a similar pattern, in which the D10 values of planktonic cells (0.653 kGy) were significantly higher than those for biofilm cells (0.479 kGy), while isolate 35150 showed the reverse, with D10 values of planktonic cells (0.396 kGy) significantly lower than those for biofilm cells (0.526 kGy). At the 48-h and 72-h sampling times, there were no differences in radiation sensitivities based on biofilm habitat for C9490 or 35150. Biofilm-associated cells, therefore, show a response to irradiation which can differ from that of planktonic counterparts, depending on the isolate and the culture maturity. Culture maturity had a more significant influence on the irradiation efficacy of planktonic cells but not on biofilm-associated cells of E. coli O157:H7.
Escherichia coli O157:H7 is a food-borne pathogen associated with a variety of food products, including meats, leafy vegetables, sprouts, and other foods (1, 13, 14, 17, 21). E. coli O157:H7 and other human pathogens such as Staphylococcus aureus, Salmonella spp., and Listeria monocytogenes are known to adhere to food surfaces and to inert surfaces such as stainless steel, plastic, glass, and cement (4, 5, 9, 10, 18, 19). The adherence and subsequent formation of biofilms, or the participation of these pathogens in biofilms crated by other organisms, can serve as a source of contamination on foods and in food processing environments.
Biofilms are organized, structured communities of bacteria living at a liquid interface that protects and supports growth and reproduction as well as communication and other interactions among the members of the population (2, 22). Exopolysaccharides (EPS) and other extracellular material within the biofilm form the complex structural matrix that effectively protects the bacterial participants from chemical sanitizers, rendering biofilm-associated bacteria more resistant to aqueous antimicrobial agents (such as trisodium phosphate, iodophor, and chlorine) than their planktonic counterparts (9, 11, 20, 22). A commonly recommended concentration of chlorine, 200 ppm, was effective against planktonic cells but ineffective when applied to biofilm-associated E. coli O157:H7 (19). Biofilm-associated Staphylococcus aureus was killed by chlorine only at levels representing a 600-fold increase over that required to kill planktonic cells (10). A similar tolerance for chlorine was observed for Salmonella, with a chlorine treatment regimen sufficient to kill 6 log units of planktonic cells (10 ppm, 10 min), resulting in less than 1 log unit reduction of biofilm-associated cells (9).
Irradiation effectively eliminates spoilage and pathogenic microorganisms in foods (14, 21). The radiation dose value required to reduce the population by one log or 90% (D10) for E. coli O157:H7 depends on the isolate, the suspending food product, and the conditions of treatment (13, 14, 21). When inoculated onto fresh sprouts of radish, alfalfa, or broccoli seeds, E. coli O157:H7 showed a D10 value range of 0.27 to 0.34 kGy, depending on the type of sprout examined (17). On different types of lettuce, D10 values of between 0.12 and 0.14 kGy were reported, depending on the suspending type of lettuce (13). The D10 value of E. coli O157:H7 typically ranges from 0.11 to 0.5 kGy on foods (1, 14, 21).
In a direct comparison, biofilm-associated Salmonella cells were recently shown to be either similarly susceptible or more susceptible to ionizing radiation than corresponding planktonic cells (15). This result stands in contrast to the protective effect that the biofilm habitat affords against other antimicrobial processes but is not sufficient information for a generalized understanding of the effect of the biofilm habitat on the radiation sensitivity of biofilm-associated cells.
Irradiation acts via radical molecules created when high-energy particles split water molecules within the product or suspending environment and within the resident bacteria (14). These radicals damage cellular and biochemical structures that they come in contact with, such as nucleic acid strands, cell membranes, and protein structures. Under conditions of limited free water, such as in dry or frozen products, the mobility of these radicals is limited, increasing the likelihood of the radicals self-quenching rather than damaging the nearby bacterial cells. In these protective environments, higher doses of irradiation are required to effect equivalent log reductions of target pathogens, resulting in higher D10 values (14, 21). In discussing potential mechanisms by which the biofilm habitat might either increase or decrease the effectiveness of irradiation against biofilm-associated bacteria, Niemira (14) speculated that radical formation is most likely not hindered in a biofilm, due to the water-saturated nature of the EPS matrix, but mobility of the radicals may be hindered, leading to self-quenching and reduced efficacy. It was further speculated that the structural elements of the EPS matrix may themselves be the primary targets of the radicals, thereby protecting the associated bacteria. The extent to which a biofilm-derived EPS matrix influences the efficiency of radical formation or influences the mobility of radicals or is itself damaged by reaction with radiation-induced radicals is unknown.
Bacterial cells which are suspended in solutions with a relatively high antioxidant capacity are more protected from irradiation due to the radical scavenging ability of the suspending solution (21). Depending on the physiology of the microbial community that forms the biofilm, the matrix may include exogenously held compounds with antioxidant capacity, an additional mechanism by which the biofilm milieu could reduce the efficacy of irradiation (14). The chemical composition of the EPS matrix, with respect to its antioxidant or radical quenching capacity, and how this may be influenced by isolate and/or culture conditions are not yet known.
The goals of this study were to investigate the relative susceptibilities of planktonic cells versus those of biofilm-associated cells of three isolates of E. coli O157:H7 to irradiation and to determine the significance of the maturity of the biofilm in the antimicrobial efficacy of the treatment.
MATERIALS AND METHODS
Bacteria.
All isolates utilized in this study were from the USDA-ARS-ERRC culture collection. Stock cultures of E. coli O157 isolates ATCC 35150, ATCC 43894, and C9490 (CDC) were stored in tryptic soy broth (TSB; Difco, Detroit, MI) containing 30% glycerol at −80°C. Working cultures were maintained on tryptic soy agar (TSA; Difco, Detroit, MI) slants at 4°C.
Preparation of biofilms.
Biofilm production methodologies vary in terms of incubation times and temperatures, media, medium changes, etc., depending on the intent of the study (11). Given the relative paucity of literature addressing the question of irradiation of biofilm-associated human pathogens, the methods used herein for biofilm cultivation, irradiation, and enumeration were chosen to facilitate comparisons with previously published information (15). Planktonic and biofilm-associated cells were prepared in sterile 50-ml centrifuge tubes according to the method of Niemira and Solomon (15). Briefly, sterile TSB (10 ml) was inoculated with 200 μl of a stock TSB culture and grown overnight at 37°C to make a stock culture (approximately 109 CFU/ml) which was used to inoculate the biofilm coincubation apparatus. Previous experience with these isolates indicates that stationary phase is reached in agitated TSB at approximately 24 h of incubation at 37°C. Precleaned glass microscope slides, 7.62 cm by 2.54 cm, were wrapped in foil and sterilized by autoclaving (121°C, 15 min) using a gravity cycle. Slides were aseptically placed into 50-ml tubes containing 25 ml of sterile TSB and inoculated with 200 μl of the stock culture. Tubes were held upright in a rack and incubated at 37°C for 24 h, 48 h, or 72 h under static conditions. This configuration resulted in approximately 3.5 cm of the slide submerged in culture medium; the upper part of the slide in the headspace of the tube remained dry. The total area for biofilm formation was approximately 17.8 cm2 (2 by 3.5 cm by 2.54 cm). The studies were performed in triplicate. Noninoculated negative control tubes were prepared similarly and incubated to identify potential cross-contamination of experimental tubes. Replications with contaminated control tubes were discarded in toto and repeated. For each replication, separate stock cultures were grown, and separate coincubation tubes were inoculated, irradiated concurrently, and sampled individually (as described below).
Irradiation.
Samples were irradiated using a Lockheed-Georgia (Marietta, GA) cesium-137 temperature-controlled gamma radiation source, with a dose rate of 5.22 kGy/h, according to the method of Niemira et al. (13). This irradiator incorporates an annular array of steel tubes containing the gamma-emitting material (“pencils”), arranged vertically around the treatment chamber, ensuring dose uniformity. The sample tubes of the separate, duplicate replicates were irradiated concurrently. The sample tubes were given doses ranging from 0.0 to 1.5 kGy. The temperature was held constant at 22°C during irradiation by the injection of gas-phase liquid nitrogen into the top of the chamber. Alanine pellets (Bruker, Inc., Billerica, MA) were used for dosimetry. The pellets were placed in dry tubes in the same rack as the culture tubes prior to irradiation and irradiated concurrently. The dosimeters were read on a Bruker EMS 104 electron paramagnetic resonance (EPR) analyzer and compared with a previously determined standard curve. The delivered dose, as determined by EPR dosimetry, was typically within 5% of the nominal dose.
Enumeration of survivors.
Tubes were opened and surviving planktonic cells were enumerated by withdrawing a 100 μl aliquot of the liquid culture, serial dilution with Butterfield's phosphate buffer (BPB; Hardy Diagnostics, Santa Maria, CA), and spread plating using TSA. In withdrawing the sample of liquid culture containing planktonic cells, extreme care was taken to avoid contacting either the glass slide or the sides of the culture tube. Biofilm-associated cells were isolated and enumerated according to the method of Niemira and Solomon (15). The microscope slide was carefully removed using sterile forceps to grip the clean, dry upper portion of the slide and rinsed for 10 seconds under a stream of sterile distilled water to remove unattached cells. The slide was then vigorously shaken in 25 ml of BPB in a fresh, sterile 50-ml centrifuge tube. This method has been shown to effectively remove essentially all of the biofilm from the glass slides used in this system (15). All samples, planktonic and biofilm, were thoroughly vortexted prior to dilution and again after every step in the dilution series. Aliquots were then spread plated using TSA. Plates were incubated overnight at 35°C and counted by hand. Based on preliminary studies, radiation doses and serial dilutions were chosen to provide plate counts of between 20 and 300 CFU/plate.
Statistical analysis.
Data were normalized against the control and plotted as the log reduction required to produce a survivor ratio. The data were pooled, and the slopes of the individual survivor curves were calculated with linear regression (SigmaPlot 5.0; SPSS Inc., Chicago, IL) and compared using analysis of covariance (ANCOVA; Excel 97, Microsoft Corp., Redmond, WA). ANCOVA incorporates the measurement of variance of the data in establishing the significance of differences (P < 0.05) of slopes of lines. The ionizing radiation D10 value was calculated by taking the negative reciprocal of the survivor curve slope.
RESULTS AND DISCUSSION
Ionizing radiation effectively reduced the populations of E. coli O157:H7 isolates C9490 (Fig. 1), 43894 (Fig. 2), and 35150 (Fig. 3). This reduction was dose related and was evident for both planktonic and biofilm-associated cells at all cultivation times. Populations of nontreated controls (0.0 kGy) were consistent for all isolates and sampling times, as follows: 8.82 to 9.37 log CFU/ml for planktonic cells and 6.59 to 8.03 log CFU/ml for biofilm-associated cells (Table 1). This suggests that a consistent stationary phase of population was reached at or around 24 h, and as there was no diminution in CFU/ml by 72 h, the death phase had not been reached by that latest time of cultivation. This point is further discussed below. Plate counts are able to establish the viable population numbers, but they are not able to provide information on the more subtle aspects of physiological activity of the older cultures versus that of the younger. It should be noted that although the recovery procedure has been previously shown to effectively remove essentially all of the biofilm from the glass slides used in this apparatus, it is possible that the vigorous shaking and repeated vortexing may not have completely disrupted all of the biofilm material. Some percentage of the resulting colonies may therefore have been derived from “clumps” of biofilm. While the generally consistent plate counts from the biofilm samples suggest that this did not play a significant factor in the enumeration, this is an area in which the methods used herein may benefit from further development. Future research may incorporate modified medium strength and/or composition, sequential medium changes, or other methodologies which can serve to elucidate the phenomena in question.
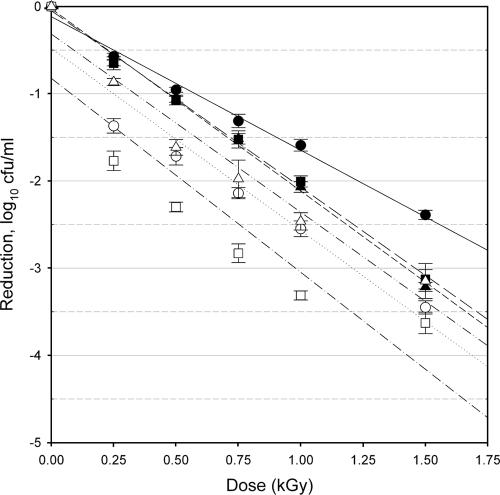
FIG. 1.
Response of E. coli O157:H7 isolate C9490 to irradiation treatments. Planktonic cells (filled symbols) and biofilm-associated cells (open symbols) were evaluated after 24 h (circles), 48 h (squares), and 72 h (triangles) of cultivation. Bars indicate standard errors, n = 9 for each point of measurement. Lines indicate the linear regression for planktonic cells at 24 h (solid line), 48 h (long-dash line) or 72 h (short-dash line); biofilm-associated cells at 24 h (dotted line), 48 h (dash-single-dot line), or 72 h (dash-double-dot line).
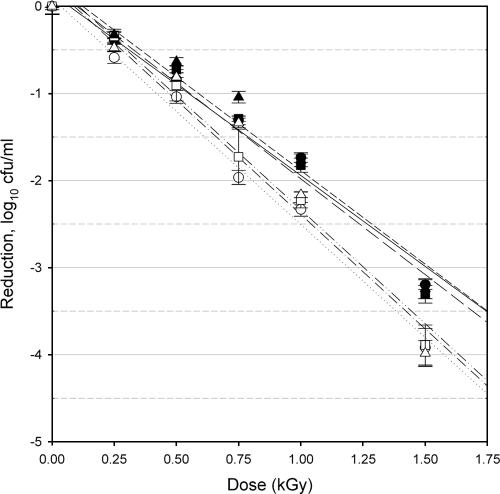
FIG. 2.
Response of E. coli O157:H7 isolate 43894 to irradiation treatments. Planktonic cells (filled symbols) and biofilm-associated cells (open symbols) were evaluated after 24 h (circles), 48 h (squares), and 72 h (triangles) of cultivation. Bars indicate standard errors, n = 9 for each point of measurement. Lines indicate the linear regression for planktonic cells at 24 h (solid line), 48 h (long-dash line), or 72 h (short-dash line); biofilm-associated cells at 24 h (dotted line), 48 h (dash-single-dot line), or 72 h (dash-double-dot line).
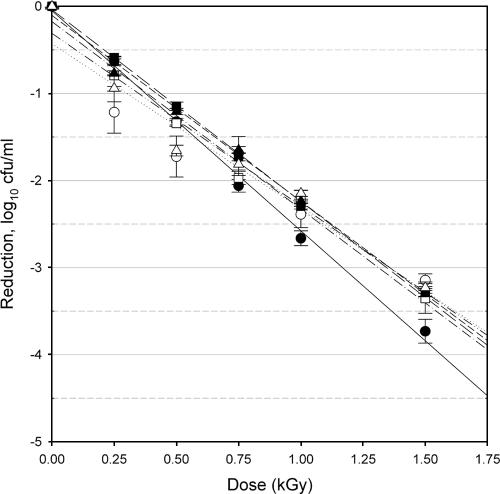
FIG. 3.
Response of E. coli O157:H7 isolate 35150 to irradiation treatments. Planktonic cells (filled symbols) and biofilm-associated cells (open symbols) were evaluated after 24 h (circles), 48 h (squares), and 72 h (triangles) of cultivation. Bars indicate standard errors, n = 9 for each point of measurement. Lines indicate the linear regression for planktonic cells at 24 h (solid line), 48 h (long-dash line) or 72 h (short-dash line); biofilm-associated cells at 24 h (dotted line), 48 h (dash-single-dot line), or 72 h (dash-double-dot line).
TABLE 1.
Radiation D10 values for planktonic and biofilm-associated cells of E. coli O157:H7 isolates C9490, 43894, and 35150
| Isolatea | Isolate culture ageb
|
Maturity P valuesc | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h
|
48 h
|
72 h
|
|||||||||||
| CFU/ml (±SD) | D10 (kGy) | r2 value | P value significance | CFU/ml (±SD) | D10 (kGy) | r2 value | P value significance | CFU/ml (±SD) | D10 (kGy) | r2 value | P value significance | ||
| C9490-P | 9.18 ± 0.08 | 0.653 | 0.96 | A | 9.28 ± 0.08 | 0.494 | 0.95 | A | 9.26 ± 0.04 | 0.478 | 0.98 | A | * |
| C9490-B | 7.04 ± 0.28 | 0.479 | 0.90 | B | 7.61 ± 0.40 | 0.442 | 0.80 | ACD | 7.00 ± 0.47 | 0.490 | 0.84 | A | NSD |
| 43894-P | 9.08 ± 0.11 | 0.479 | 0.97 | B | 8.82 ± 0.65 | 0.454 | 0.96 | AC | 8.98 ± 0.17 | 0.465 | 0.93 | A | NSD |
| 43894-B | 8.03 ± 0.90 | 0.385 | 0.94 | C | 7.62 ± 0.31 | 0.381 | 0.85 | D | 7.74 ± 0.30 | 0.382 | 0.94 | B | NSD |
| 35150-P | 9.08 ± 0.05 | 0.396 | 0.97 | C | 9.29 ± 0.06 | 0.454 | 0.99 | BC | 9.37 ± 0.05 | 0.469 | 0.99 | A | * |
| 35150-B | 6.59 ± 0.24 | 0.526 | 0.74 | B | 6.75 ± 0.31 | 0.464 | 0.92 | AB | 6.88 ± 0.17 | 0.503 | 0.90 | A | NSD |
Isolates were evaluated as planktonic cells (suffix “P”) or as biofilm-associated cells (suffix “B”).
Observed log CFU/ml populations are shown for control (0.0 kGy) samples, mean of 9 samples, plus or minus standard deviations (SD). r2 values of the linear regression model were used to calculate D10 values. D10 values for a given time of cultivation shown with the same letter (A, B, C, and D) are not significantly (P < 0.05) different (by analysis of covariance).
D10 values for a given isolate/cultivation method are either significantly different (*, P < 0.05) or not significantly different (NSD) across the three times of cultivation (by analysis of covariance).
The radiation D10 values for planktonic cells ranged from 0.396 to 0.653 kGy, while the D10 values for biofilm-associated cells ranged from 0.381 to 0.526 kGy (Table 1). These values are in agreement with previously published D10 values for E. coli O157:H7 in/on a variety of food products (13, 14, 21). There were significant differences in the observed radiation resistances related to the isolate, the maturity of the culture, and the growth form evaluated (planktonic versus biofilm associated).
Isolate specificity.
The planktonic cells of the three strains of E. coli O157:H7 used in this study showed significantly different (P < 0.05) resistance values to irradiation in the 24-h and 48-h samples but were not significantly different from each other in the 72-h sampling. At all sampling times, the D10 of biofilm-associated cells of isolates C9490 and 35150 were not different from each other. In contrast, at all sampling times, the D10 of biofilm-associated cells of isolate 43894 were significantly lower than those of either C9490 or 35150, indicating that the biofilm-associated cells of this isolate were consistently more sensitive to irradiation. The D10 values of 43894 ranged from 14 to 27% less than those of the other two strains. Strain-dependent response to irradiation is a well-documented phenomenon and has been previously observed with E. coli O157:H7 irradiated on a variety of food and nonfood substrates (14, 17, 21, 24). However, this is the first direct examination of the role of isolate specificity with regard to the radiation sensitivity of biofilm-associated E. coli O157:H7 cells. The mechanisms responsible for isolate-specific differences in sensitivity to ionizing radiation are complex and have not yet been fully elucidated.
Culture maturity.
The planktonic cells of isolate C9490 were significantly (P < 0.05) more resistant to irradiation in the 24-h culture than in the 48-h and 72-h cultures, values of which did not differ from each other (Table 1). In contrast, the planktonic cells of isolate 35150 were significantly more sensitive to irradiation in the 24-h culture than in the 48-h and 72-h cultures, values of which did not differ from each other (Table 1). There was no significant effect of culture maturity on the radiation resistance of planktonic cells of isolate 43894 nor on the radiation resistance of biofilm-associated cells for any of the three isolates examined. Due to its mode of action (generation of reactive radical species), the efficacy of ionizing radiation is typically enhanced when applied to active bacterial cultures and typically reduced when applied to quiescent bacterial cultures (21). While it may therefore be expected that older cultures, which are less active, would show a reduced efficacy of irradiation (with concomitantly higher D10 values), this was not generally observed in this study. This supports the conjecture that the biofilm cultures reached a stationary stage at or shortly before 24 h, while the planktonic cultures became stationary at or shortly after 24 h. Culture age did not exert a consistent influence on the efficacy of the irradiation in the case of the planktonic cells and did not appear to have any significance in the case of biofilm cultures. These results indicate that the biofilm phenotype, a putative metabolic condition of cells associated with a mature biofilm in which susceptibility to antimicrobial agents is reduced (22), is either fully established by 24 h (and remains invariant in the 24- to 72-h time frame) or is not relevant with regard to ionizing radiation.
Growth form.
For each of the isolates tested, the radiation resistance of planktonic cells was significantly (P < 0.05) different from that of biofilm-associated cells in the 24-h culture (Table 1). For isolates C9490 and 43894, planktonic cells had D10 values that were, respectively, 36% and 24% higher than the D10 of the biofilm-associated cells. For isolate 35150, the reverse was observed, with the D10 of the biofilm-associated cells 33% higher than that of the planktonic cells. With more mature cultures, the D10 values of biofilm-associated and planktonic cultures of isolates C9490 and 35150 were no longer significantly different from each other. As the D10 value of the biofilm-associated cells was not significantly variable due to culture maturity, the change in relative radiation resistance was due to the change in the D10 values observed for planktonic cells. In contrast, however, the differences in radiation sensitivity related to growth form persisted for isolate 43894 regardless of culture maturity, with D10 values of planktonic cells higher than those of biofilm-associated cells by 24%, 19%, and 22% at the 24-h, 48-h and 72-h sampling periods, respectively. For this isolate, the D10 values for both planktonic and biofilm-associated cells were consistent with culture maturity. This suggests a more fundamental difference between cells in the two growth forms for isolate 43894 than for the other two.
The response of biofilm-associated pathogens to irradiation is largely unexplored and is in any event poorly understood. Information is available on the efficacy of ionizing radiation in eliminating pathogens attached to foods and food surfaces (1, 13, 14, 21, 24). Niemira et al. (13) reported D10 values of 0.12 to 0.14 kGy for E. coli O157:H7 on lettuce leaves. Grant and Patterson (7) reported D10 values ranging from 0.371 to 0.697 kGy for S. enterica serovar Typhimurium in components of a roast beef ready meal. D10 values of between 0.374 and 0.773 were reported for six strains of Salmonella when irradiated in mechanically deboned chicken meat (24). Clavero et al. (1) found D10 values ranging from 0.618 to 0.800 kGy for salmonellae in raw ground beef, depending on irradiation temperatures and fat content. However, the extent to which these studies were evaluating the response of bacteria as part of a true biofilm is uncertain. This distinction is important due to the well-documented negative impact of the biofilm habitat, with its protective exopolysaccharide matrix, on the efficacy of many antimicrobial processes (2, 19, 22).
In a study which involved a direct comparison, Niemira and Solomon (15) irradiated the planktonic and biofilm-associated cells of three Salmonella isolates and showed that the biofilm-associated cells of S. enterica serovar Stanley and serovar Enteritidis were significantly more sensitive to ionizing radiation than respective planktonic cells. The third isolate examined in that study, serovar Anatum, showed no increase in radiation sensitivity for biofilm-associated cells. The authors concluded that the antimicrobial efficacy of ionizing radiation is therefore preserved or enhanced when treating biofilm-associated bacteria. It should be noted that all of the cultures examined in that study were the same age, 48 h (15). The extent to which the response of Salmonella planktonic cultures might vary with maturity, as do the E. coli O157:H7 cultures examined herein, or with the potential for differential response of Salmonella biofilms of various maturities, remains unknown.
The primary mode of action of irradiation is via oxygen and hydroxyl radicals (14, 21). Solutions with a high antioxidant capacity are known to protect suspended bacteria by neutralizing these radicals before they can damage bacterial cell membranes or other structures, thereby reducing the efficacy of the process (21). The biofilm EPS microstructural elements have been proposed as a mechanism by which biofilm-associated pathogens either might be protected from irradiation or might be a source of hydroxyl radicals, thereby enhancing the antimicrobial effect (14). In the case of isolate 43894, the biofilm-associated cells appeared to be more sensitive than their planktonic counterparts. As the responses of C9490 and 35150 were more variable, definite conclusions regarding the influence of the EPS matrix on in situ radiation chemistry cannot be made without more detailed information about the specific nature of the different matrices produced by these isolates. The data obtained remain insufficient to definitively address speculations regarding the extent to which EPS-associated elements such as DNA, proteins, etc., might inhibit the generation, mobility, or quenching of radical molecules throughout the biofilm. Further research may also address the possibility of the formation of chemical intermediates with antimicrobial properties, such as peroxides, within or near the biofilm as a result of irradiation. It remains an open question whether such endogenously created chemical intermediaries would mimic the behavior of exogenously applied antimicrobials, with respect to diffusion, migration, efficacy, etc.
In general, despite the variation in D10 values obtained, the observed antimicrobial efficacy of the process against biofilm-associated pathogens indicates that irradiation remains an effective means of inactivating bacteria, even within a biofilm. The postirradiation behavior of sublethally injured biofilm-associated bacteria is poorly understood. The possibility exists for regrowth of the pathogen and recolonization of the surface. In discussing the postirradiation biofilm environment, Niemira (14) speculated that the close proximity of different bacterial species with different levels of native radiation resistance may lead to postirradiation competition for resources that places additional pressure on radiation-injured bacteria. However, the EPS matrix may also provide for enhanced recovery from radiation injury by protecting the bacteria from desiccation injury or from competition with organisms outside the biofilm. The extent to which quorum sensing behaviors might influence postirradiation recovery or recolonization of surfaces by pathogens, singly or in a complex biofilm, is a key area for future research. It is possible that the interruption of quorum sensing may serve to heighten the antimicrobial efficacy of the process, although data to support this supposition are lacking. It should be stressed that the biofilm ecologies of foods and food contact surfaces are complex and dynamic. The postirradiation response of the various pathogenic and nonpathogenic members of the biofilm community, in competition, predation, and other interactions remains unexplored (11, 12, 22). More research is required to more completely understand the details of postintervention population dynamics, to define the behavior of irradiated biofilms.
In the present study, the efficacy of irradiation of biofilm-associated versus planktonic cells varied significantly but by less than an order of magnitude, either in reduction or enhancement. It has been repeatedly demonstrated that the efficacy of aqueous sanitizers is reduced when applied to biofilm-associated bacteria, often by several orders of magnitude (19). Chlorine was shown to penetrate only poorly into biofilms of Klebsiella pneumoniae and Pseudomonas aeruginosa, with concentrations within the biofilm reduced 80% or more, relative to that in the bulk fluid (3). As increased contact time did not increase penetration in that study, it was concluded that the lack of penetration is a primary mechanism for the reduced efficacy of antimicrobials when applied against biofilms (2, 3). Biofilms of Listeria monocytogenes showed an exceptional tolerance for sodium hypochlorite treatment, with only a 2-log reduction following a treatment that was significantly more aggressive (1,000 ppm, 20 min) than that which yielded an 8-log reduction of planktonic cells (10 ppm, 0.5 min) (16). Treatment with alkaline hypochlorite, chlorsulfamates, and trisodium phosphate yielded similar results (20, 23).
The structural organization of biofilms hinders or blocks the introduction of diffusible, antimicrobial chemicals. Processes which are nondiffusive but which, instead, rely on energy transfer may therefore have greater potential when directed specifically against biofilm-associated pathogens. Examples of these processes include ionizing radiation, UV irradiation (8), and radio frequency treatments (6). However, as biofilm-associated bacteria have also demonstrated enhanced resistance to heat treatment (5), it is necessary to determine the mechanisms by which these processes inactivate the target organisms. The radiation D10 values obtained in the present study demonstrate that despite the significant differences resulting from isolate, growth form, and/or culture age, the biofilm habitat's previously established protective effect does not extend to treatment with ionizing radiation. Irradiation is, therefore, shown to be an effective antimicrobial intervention against biofilm-associated as well as planktonic E. coli O157:H7 cells. The extent to which irradiation or other energy transfer interventions may be combined, either simultaneously or sequentially, with antimicrobial chemical treatments is a subject of ongoing research.
Summary.
Irradiation effectively reduces the population of both planktonic and biofilm-associated E. coli O157:H7. The radiation sensitivity is isolate specific, as is the influence of time of cultivation on the response to irradiation of planktonic cells. Similarly, the influence of the cultured state of the organism, i.e., planktonic versus biofilm associated, is also isolate specific. In contrast to chemical antimicrobial treatments that can show a 10-fold or 100-fold diminution of efficacy when directed against biofilm-associated pathogens, the radiation D10 value for biofilm-associated pathogens was increased for only one isolate (35150) at one sampling time (24 h). All other isolate/time combinations showed either no change in D10 value or an increased efficacy of irradiation against biofilm-associated pathogens.
Acknowledgments
I thank X. Fan and T. Z. Jin for their thoughtful reviews of the manuscript and C. M. Clark, L. Cheung, and J. S. Taylor for technical assistance.
Mention of trade names and commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 23 March 2007.
REFERENCES
- 1.Clavero, M. R. S., J. D. Monk, L. R. Beuchat, M. P. Doyle, and R. E. Brackett. 1994. Inactivation of Escherichia coli O157:H7, salmonellae, and Campylobacter jejuni in raw ground beef by gamma irradiation. Appl. Environ. Microbiol. 60:2069-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies, D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. 2:114-122. [DOI] [PubMed] [Google Scholar]
- 3.de Beer, D., R. Srinivasan, and P. S. Stewart. 1994. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Environ. Microbiol. 60:4339-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhir, V. K., and C. E. R. Todd. 1995. Susceptibility of suspended and surface-attached Salmonella enteritidis to biocides and elevated temperatures. Appl. Environ. Microbiol. 61:1731-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank, J., and R. Koffi. 1990. Surface-adherence growth of Listeria monocytogenes is associated with increased resistance to surface sanitizers and heat. J. Food Prot. 53:550-554. [DOI] [PubMed] [Google Scholar]
- 6.Geveke, D. J., M. F. Kozempel, O. J. Scullen, and C. Brunkhorst. 2002. Radio frequency energy effects on microorganisms in foods. Innov. Food Sci. Emerg. Technol. 3:133-138. [Google Scholar]
- 7.Grant, I. R., and M. F. Patterson. 1992. Sensitivity of foodborne pathogens to irradiation in the components of a chilled ready meal. Food Microbiol. 9:95-103. [Google Scholar]
- 8.Guerrero-Beltran, J. A., and G. V. Barbossa-Canovas. 2004. Review: advantages and limitations on processing foods by UV light. Food Sci. Tech. Int. 10:137-147. [Google Scholar]
- 9.Joseph, B., S. K. Otta, I. Karunasagar, and I. Karunasagar. 2001. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int. J. Food Microbiol. 64:367-372. [DOI] [PubMed] [Google Scholar]
- 10.Luppens, S. B. I., M. W. Reij, R. W. L. van der Heijden, F. M. Rombouts, and T. Abee. 2002. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 68:4194-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLean, R. J. C., C. L. Bates, M. B. Barnes, C. L. McGowin, and G. M. Aron. 2004. Methods of studying biofilms, p. 379-413. In M. Ghannoum and G. A. O'Toole (ed.), Microbial biofilms. ASM Press, Washington, DC.
- 12.Morris, C. E., J.-M. Monier, and M.-A. Jaques. 1997. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl. Environ. Microbiol. 63:1570-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niemira, B. A., C. H. Sommers, and X. Fan. 2002. Suspending lettuce type influences recoverability and radiation sensitivity of Escherichia coli O157:H7. J. Food Prot. 65:1388-1393. [DOI] [PubMed] [Google Scholar]
- 14.Niemira, B. A. 2003. Irradiation of fresh and minimally processed fruits, vegetables and juices, p. 279-300. In J. S. Novak, G. M. Sapers, and V. K. Juneja (ed.), The microbial safety of minimally processed foods. CRC Press, Boca Raton, FL.
- 15.Niemira, B. A., and E. B. Solomon. 2005. Sensitivity of planktonic and biofilm-associated Salmonella spp. to ionizing radiation. Appl. Environ. Microbiol. 71:2732-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norwood, D. E., and A. Gilmour. 2000. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J. Appl. Microbiol. 88:512-520. [DOI] [PubMed] [Google Scholar]
- 17.Rajkowski, K. T., and D. W. Thayer. 2000. Reduction of Salmonella spp. and strains of Escherichia coli O157:H7 by gamma radiation of inoculated sprouts. J. Food Prot. 63:871-875. [DOI] [PubMed] [Google Scholar]
- 18.Reisner, A., K. A. Krogfelt, B. M. Klein, E. L. Zechner, and S. Molin. 2006. In vitro biofilm formation of commensal and pathogenic Escherichia coli strains: impact of environmental and genetic factors. J. Bacteriol. 188:3572-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu, J.-H., and L. R. Beuchat. 2005. Biofilm formation by Escherichia coli O157:H7 on stainless steel: effect of exopolysaccharide and curli production on its resistance to chlorine. Appl. Environ. Microbiol. 71:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somers, E. B., J. L Schoeni, and A. C. L. Wong. 1994. Effect of trisodium phosphate on biofilm and planktonic cells of Campylobacter jejuni, Escherichia coli O157:H7, Listeria monocytogenes and Salmonella typhimurium. Int. J. Food Microbiol. 22:269-276. [DOI] [PubMed] [Google Scholar]
- 21.Sommers, C. H. 2003. Irradiation of minimally processed meats, p. 301-318. In J. S. Novak, G. M. Sapers, and V. K. Juneja (ed.), The microbial safety of minimally processed foods. CRC Press, Boca Raton, FL.
- 22.Starkey, M., K. A. Gray, S. I. Chang, and M. R. Parsek. 2004. A sticky business: the extracellular polymeric substance matrix of bacterial biofilms, p. 174-191. In M. Ghannoum and G. A. O'Toole (ed.), Microbial biofilms. ASM Press, Washington, DC.
- 23.Stewart, P. S., J. Rayner, F. Roe, and W. M. Rees. 2001. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorsulfamates. J. Appl. Microbiol. 91:525-532. [DOI] [PubMed] [Google Scholar]
- 24.Thayer, D. W., G. Boyd, W. S. Muller, C. A. Lipson, W. C. Hayne, and S. H. Baer. 1990. Radiation resistance of Salmonella. J. Ind. Microbiol. 5:387-390. [Google Scholar]