Abstract
The acidophilic proteobacterium Acidithiobacillus ferrooxidans is involved in the industrial biorecovery of copper. It is found in acidic environments in biofilms and is important in the biogeochemical cycling of metals and nutrients. Its genome contains a cluster of four genes, glyQ, glysS, gph, and act, that are predicted to encode the α and β subunits of glycine tRNA synthetase, a phosphatase, and an acyltransferase, respectively (GenBank accession no. DQ149607). act, cloned and expressed in Escherichia coli, produces acyl homoserine lactones (AHLs) principally of chain length C14 according to gas chromatography and mass spectrometry measurements. The AHLs have biological activity as shown by in vivo studies using the reporter strain Sinorhizobium meliloti Rm41 SinI−. Reverse transcription-PCR (RT-PCR) experiments indicate that the four genes are expressed as a single transcript, demonstrating that they constitute an operon. According to semiquantitative RT-PCR results, act is expressed more highly when A. ferrooxidans is grown in medium containing iron than when it is grown in medium containing sulfur. Since AHLs are important intercellular signaling molecules used by many bacteria to monitor their population density in quorum-sensing control of gene expression, this result suggests that A. ferrooxidans has two quorum-sensing systems, one based on Act, as described herein, and the other based on a Lux-like quorum-sensing system, reported previously. The latter system was shown to be upregulated in A. ferrooxidans grown in sulfur medium, suggesting that the two quorum-sensing systems respond to different environmental signals that may be related to their abilities to colonize and use different solid sulfur- and iron-containing minerals.
Quorum sensing (QS) is a process by which bacteria communicate via the secretion and detection of chemical signaling molecules termed autoinducers. It is an important method for the regulation of population density-dependent cellular processes, such as the production of antibiotics and virulence factors, conjugation, transformation, swarming behavior, and biofilm formation (20). Several different QS systems have been discovered including the LuxIR paradigm, in which the signaling molecule is an acyl homoserine lactone (AHL), used principally by gram-negative bacteria (22, 39, 40); the furanosyl-borate diester signaling system (3); the peptide signaling systems used primarily by gram-positive bacteria (7); the LuxS/autoinducer-2 signaling used for interspecies communication; and the 3/epinephrine/norepinephrine interkingdom signaling system (36).
Although QS systems have been described in a wide variety of microorganisms (26), there are few reports of the presence of QS in extremophiles. AHL-based QS has been detected in the haloalkaliphilic archaeon Natronococcus occultus (21) and in the haloalkaliphilic Halomonas genus of bacteria (17). In addition, peptide-based QS has been detected in the hyperthermophilic bacterium Thermotoga maritima (13). Little is known about QS systems in other extremophilic archaea, although a genome-wide survey indicated that a furanosyl-borate diester signaling system may be prevalent in archaea (38). The paucity of information regarding bacterial communication in extreme conditions, especially in acidic environments, prompted the present investigation.
Recently, a classic LuxIR system was described in the extreme acidophile Acidithiobacillus ferrooxidans (8, 27). A. ferrooxidans is a chemolithoautotrophic, mesophilic, facultative aerobe of the gammaproteobacterium group. It obtains energy and electrons by the oxidation of hydrogen and reduced sulfur compounds to sulfate and FeII to FeIII. It grows in extremely acidic conditions (<pH 2) and fixes CO2 and N2 to acquire cellular carbon and nitrogen. A. ferrooxidans is used for the industrial recovery of copper and gold in various parts of the world (25). It is also an important source of acid mine drainage and may play a significant role in the biogeochemical cycling of iron, sulfur, heavy metals, and nutrients in acidic environments (12). In an effort to broaden our understanding of QS in A. ferrooxidans, the complete genome was searched for candidate genes with similarity to all known genes involved in QS. We discovered an ortholog of hdtS, which encodes an AHL synthase in Pseudomonas fluorescens F113 (15), in the A. ferrooxidans genome, and we termed it act for acyl transfer function. This paper provides evidence for the role of Act in the formation of AHLs in A. ferrooxidans.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Acidithiobacillus ferrooxidans ATCC 23270 was grown in 9K salts medium (pH 2.4) supplemented with elemental sulfur or iron (FeSO4) as described previously (44). Escherichia coli JM109 was grown in Luria-Bertani (LB) medium. Agrobacterium tumefaciens NT1 was grown in LB medium containing 50 μg ml−1 kanamycin at 30°C. Sinorhizobium meliloti Rm41 was grown in LB medium at 30°C, and S. meliloti Rm41 SinI− was grown in LB medium or MMgly medium (11 g of Na2HPO4, 3 g of KH2PO4, 0.5 g of NaCl, 1 g of NH4Cl, 5 ml of glycerol, 1 mg of biotin, 27.8 mg of CaCl2, and 246 mg of MgSO4 per liter) at 30°C supplemented with 1% (wt/vol) l-arabinose. Antibiotics were added where appropriate at the following final concentrations: 200 μg ml−1 neomycin and 50 μg ml−1 gentamicin. Details of the phenotypes and sources of bacteria are shown in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype/phenotype | Reference or source |
|---|---|---|
| Strains | ||
| Acidithiobacillus ferrooxidans ATCC 23270 | Type strain | ATCC |
| Escherichia coli JM109 | F′ traD36 lacIq(lacZ)M15 proA+B+/e14(mcrA) (lac-proA) thi gyrA96(Nalr) endA hsdR17(rK mK+) relA1 supE44 recA | Promega |
| Agrobacterium tumefaciens NT1 | traR traG::lacZ; biosensor reporter; Kmr | 34 |
| Sinorhizobium meliloti | ||
| Rm41 | Type strain; wild-type AHL overproducer | 18 |
| Rm41 SinI− | sinR sinI::lacZ; biosensor reporter; Gmr Neor | 18 |
| Plasmids | ||
| pKK223-3 | Expression plasmid vector; Ampr | Pharmacia |
| pAf-act | act of A. ferrooxidans expressed from pKK223-3 tac promoter; Ampr | This study |
Bioinformatic analyses.
Candidate protein coding genes were identified in the genome sequence of A. ferrooxidans ATCC 23270, made available by The Institute for Genome Research (http://www.tigr.org) using Glimmer (http://www.tigr.org), Critica (http://www.ttaxus.com), and BlastX (http://www.ncbi.nlm.nih.gov), followed by manual curation of the predicted genes to correct errors in start site prediction and identify missing candidate genes. The annotated genome was displayed in the interactive format of Artemis (http://www.sanger.ac.uk/Software/Artemis). The following bioinformatic programs were used to further characterize candidate genes and their predicted protein products: BlastP and PsiBlast (http://www.ncbi.nlm.nih.gov), the suite of protein characterization programs available in InterproScan (http://www.ebi.ac.uk/interpro), Blocks (http://www.blocks.fhcrc.org), and ClustalW (http://www.ebi.ac.uk/ClustalW). Conserved motifs in the ClustalW alignments were visualized using Logos (4).
Purification and identification of AHL.
Recombinant E. coli pAf-act early-stationary-phase cultures (including cells and supernatants) were extracted with dichloromethane (DCM) at a ratio of 70:30 (culture to DCM) as described previously (19). DCM was removed by rotary evaporation, and the residue was reconstituted in 100 μl DCM for fractionation by Sep-Pack C18 preparative columns. Fractions were eluted with 1 ml in a gradient of methanol in water (20, 40, 60, 75, and 95%, vol/vol). Five fractions (F1 to F5) were collected, concentrated to 10 μl, and assayed for activity using the AHL assays described above. Samples (1 μl) were also injected in the splitless mode into a gas chromatography-mass spectrometry (GC/MS) system consisting of an Autosystem XL gas chromatograph (Perkin-Elmer, Boston, MA) with an MDN-5 column (Supelco, Bellefonte, PA) coupled to a Perkin-Elmer Turbo Mass mass spectrometer. Helium served as carrier gas. The mass spectrometer was operated in the electron impact ionization mode at 70 eV as described previously (32, 33). The following AHL standards were purchased from Fluka: N-hexanoyl-dl-homoserine lactone (AHL-C6), N-octanoyl-dl-homoserine lactone (AHL-C8), and N-tetradecanoyl-dl-homoserine lactone (AHL-C14).
Isolation of DNA, recombinant DNA techniques, and DNA sequencing.
DNA was isolated from A. ferrooxidans as previously described (1). The following standard recombinant DNA techniques were performed as previously described: digestion of DNA with restriction enzymes, agarose gel electrophoresis, purification of DNA fragments, PCR amplification of DNA, DNA ligation, plasmid preparation, and transformation of E. coli (29). DNA sequencing was carried out by the Sanger dideoxynucleotide method (29).
Construction of recombinant plasmids and analysis of gene expression.
act was amplified by PCR with Elongase mix as described by the manufacturer (Invitrogen) using genomic DNA as a template with the primers P8 and P9 (Table 2). Amplified act was cloned into pKK223-3 as described by the suppliers, and the resulting recombinant plasmid was termed pAf-act. pAf-act was transformed into E. coli JM109 by electroporation, and the transformants were grown on LB plates containing ampicillin (100 μg·ml−1) at 37°C.
TABLE 2.
PCR and RT-PCR primers used in this study
| Gene | Primer | Sequence (5′ to 3′) |
|---|---|---|
| hyp | P1 | AAGCTTGGGCTGGTCTTTCT |
| glyQ | P2 | ATGCCGGTAGGTGCCGGA |
| P3 | GTCCCCATAACGGCCGTCCGTG | |
| glyS | P4 | TTCCCGCGCGGGCAACTC |
| P5 | CTCGGGCTGCTCGCAGGC | |
| gph | P6 | GCCACTCTGCGGGCGATTTGAT |
| P7 | CGACCCATGCTGGTACTGACGG | |
| act | P8 | ATGATACGCAGCATCCGC |
| P9 | TCAAAGCGCTTTTGCGCAGG | |
| rimK | P10 | GCCGCTGCGCGGGGCATG |
| recA | recA1 | CCGCCAACATTTCCCGGACC |
| recA2 | ACGCCGAGGTCCACCAGTTC |
AHL reporter plate assays.
E. coli JM109 (pAf-act) was cross streaked onto X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 80 μg ml−1)-IPTG (isopropyl-β-d-thiogalactopyranoside; 40 μg ml−1)-LB medium reporter plates in the presence of A. tumefaciens NT1 (34) and S. meliloti Rm41 SinI− (18) as described by Latifi et al. (14) and Swift et al. (39). Plates were incubated overnight at 30°C. Activation of lacZ in these reporter strains was detected by the production of blue dye. As a negative control, E. coli JM109 lacking act but containing the plasmid vector pKK223-3 was also cross streaked on the same plates. As a positive control, the AHL-producing strain S. meliloti Rm41 was also cross streaked.
Gene cotranscription analysis.
Five micrograms of total RNA was isolated from late-log/early-stationary-phase cells of A. ferrooxidans and was reverse transcribed by reverse transcription PCR (RT-PCR) as described previously (10) using the DNA primers listed in Table 2. Appropriate negative and positive controls were included in each RT-PCR experiment as described previously (10).
Semiquantitative RT-PCR measurements of gene expression.
Five micrograms of total RNA was isolated from A. ferrooxidans and was reverse transcribed by PCR (RT-PCR) as described previously (10) using the following DNA primers: P8 and P9 to amplify DNA corresponding to act and recA1 and recA2 to amplify DNA corresponding to recA (Table 2). Semiquantitative PCR was carried out for one cycle of incubation at 94°C for 1 min, followed by 15, 25, and 30 cycles of 90°C for 30 s each, 64°C for 1 min, and 72°C for 1 min. PCR products were visualized by agarose gel electrophoresis as previously described (10). Densitometry measurements of DNA were quantitated using Scion Image for Windows software. Appropriate negative and positive controls were included in each RT-PCR experiment as described previously (10). PCR was performed with up to 100-fold dilutions of template to ensure that assays were carried out in the linear range of template concentrations. Reproducibility was assessed by performing at least two independent RT reactions for each time point and at least three PCRs using each of these templates.
Nucleotide sequence accession numbers.
The nucleotide sequence of the act locus has been assigned the GenBank accession number DQ149607. The sequence of act was determined and deposited in GenBank under accession number AAZ78229.
RESULTS
The genome of A. ferrooxidans was searched for genes with similarity to all known genes involved in QS, and apart from the previously reported luxIR gene pair (8, 27) a significant hit was found only to hdtS (gi.11120074) of P. fluorescens F113 (15). hdtS has been shown to encode an AHL synthase responsible for the production of AHLs of various chain lengths. It has no significant similarity to the LuxI family of AHL synthases (15). The ortholog of hdtS in A. ferrooxidans was termed act (acyl transfer function). Predicted characteristics, including the identification of domains, motifs, and patterns of Act, are shown in Table 3.
TABLE 3.
Predicted properties of Act
| Property | Value or characteristic |
|---|---|
| Gene name (GenBank | |
| accession no.) | act (AAZ78229) |
| Predicted function | Family of 1-acyl-sn-glycerol 3-phosphate O-acyltransferases |
| Best BlastP hit | Nitrosococcus oceani |
| % Similarity | 59 |
| E value | 4e-47 |
| Score | 190 |
| Domain, motif, pattern | PF01553 SM00563 PS50239 PTHR10434 IPR002123 |
A ClustalW alignment of Act with the top 10 BlastP hits in GenBank permitted the identification of the amino acid sequence conservation of this group of proteins; in particular it highlighted the NHQS and PEGTR motifs characteristic of the family of lysophosphatidic acid (LPA) acyltransferases previously identified (42) (data not shown). The alignment showed that the first of these motifs can now be better described as KHQSAWET and that both motifs are flanked by conserved hydrophobic amino acids. Given the overall amino acid sequence conservation and taking into account the conservation of key motifs, it is predicted that Act is an acyltransferase of the family of 1-acyl-sn-glycerol 3-phosphate O-acyltransferases or so-called LPA acyltransferases (EC 2.3.1.51). This family includes members that catalyze the conversion of LPA to phosphatidic acid. Phosphatidic acid is a crucial phospholipid intermediate in cell membrane biosynthesis.
Organization and expression of a gene cluster containing act.
Analysis of the genome of A. ferrooxidans revealed the presence of a cluster of three genes, named glyQ, glyS, and gph, associated with act (Fig. 1). GlyQ and GlyS are predicted to correspond to the conserved α and β subunits of glycyl-tRNA synthetase (class II, heterodimer family, EC 6.1.1.14), respectively. Gph (general function phosphatase) shares some sequence similarity to the HisB family of histidinol-phosphate phosphatases (EC 3.1.3.15); however, it lacks the diagnostic two-domain structure of this group, and we have assigned it only a general phosphatase function at the present time.
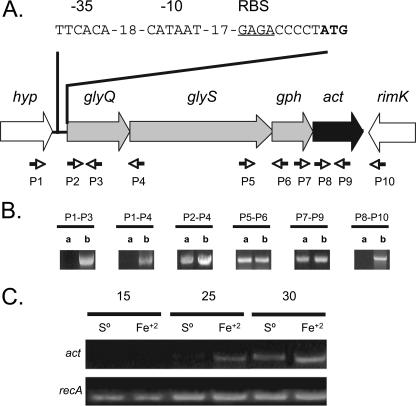
FIG. 1.
Organization and coexpression of the act locus of A. ferrooxidans and differential expression of act. (A) Organization of the act locus. Shaded arrows indicate the direction of transcription. Small black arrows indicate the position and orientation (5′ to 3′) of primers used in RT-PCR or PCR studies. A predicted sigma-70-like promoter (−35 and −10 regions separated by 18 bp), a possible ribosome binding site (RBS; underlined), and the proposed ATG initiation codon (bold) are indicated. hyp, conserved hypothetical gene of unknown function; rimK, predicted ribosomal protein S6 modification protein. (B) Coexpression of the act locus. Results of RT-PCR (using RNA as a substrate [columns a]) and PCR experiments (using genomic DNA as s substrate [columns b]) are shown. The nucleotide sequence of the act locus has been deposited in GenBank under accession number DQ149607. (C) Differential expression of act when cells were grown in either iron-containing (Fe2+) or sulfur-containing (S0) medium as determined by semiquantitative RT-PCR. The numbers refer to the numbers of PCR cycles performed. The expression of the constitutively expressed recA is included as a control.
Analysis of the act gene cluster reveals that there is an overlap of 15 bp between glyQ and glyS, a space of 3 bp between glyS and gph, and an overlap of 3 bp between gph and act. This intergenic spacing suggests that the cluster is an operon (Fig. 1A). The act operon is preceded upstream by a hypothetical conserved gene of unknown function and is followed downstream by rimK, oriented in the opposite direction from the act locus. rimK is predicted to encode a ribosomal S6 protein modification enzyme. A possible ribosome binding site with the sequence GAGA was detected 5 nucleotides upstream of the predicted ATG start site of glyQ (Fig. 1A) that shows sequence similarity to the A. ferrooxidans consensus GAGGA (unpublished results). A predicted sigma-70-like promoter was found 26 nucleotides upstream of the ATG start site with the sequence TTCACA (−35 box)-18 bp-CATAAT (−10 box) with sequence similarity to the A. ferrooxidans consensus sigma-70-like promoter (TTGACA [−35 box]-18 bp-TATAAT) (unpublished results). The intergenic spacing and the presence of a predicted promoter are consistent with the idea that the act locus is an operon. In order to evaluate this possibility, RT-PCR experiments were undertaken using primers designed for each of the predicted genes. The results indicate that glyQ, glyS, gph, and act are cotranscribed as a single message whereas hyp and rimK are not transcribed as part of the same message, indicating that the act locus is an operon (Fig. 1B).
act shows increased expression when A. ferrooxidans is grown in iron-containing medium rather than sulfur-containing medium.
RNA was isolated from cultures of A. ferrooxidans grown in either 9K medium supplemented with sulfur or the same medium supplemented with iron at pH 2. The RNA was amplified by RT-PCR using primers specific for act and using different numbers of cycles of amplification. The RT-PCR products were visualized by agarose gel electrophoresis (Fig. 1C). As a control, RT-PCR amplification was simultaneously carried out using primers specifically designed for recA. recA expression is independent of the growth medium of A. ferrooxidans (16). There is more act-specific RT-PCR product after 25 and 30 cycles of PCR amplification using RNA from cells grown in medium supplemented with iron than after the same procedure using RNA from cells grown in medium supplemented with sulfur, suggesting that there are more transcripts of act in cells grown in iron.
In vivo gene reporter assay for AHL production.
Since HdtS has been shown elsewhere to exhibit AHL synthase activity (15), it was decided to test Act for similar function. Unique primers were designed for act, and PCR was used to amplify a DNA fragment corresponding to the predicted coding region. This DNA fragment was cloned into the expression vector pKK223-3, and the resulting recombinant plasmid was termed pAf-act. The sequence of act was determined and deposited in GenBank under accession number AAZ78229.
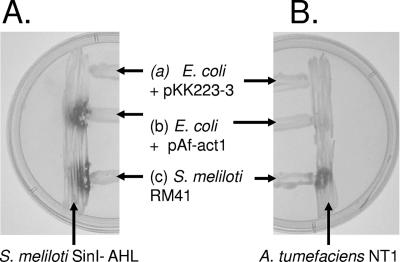
E. coli JM109 containing pAf-act was streaked onto X-Gal indicator agar plates in juxtaposition to two sensitive bacterial reporter strains of AHLs, Sinorhizobium meliloti Rm41 SinI− (18) and Agrobacterium tumefaciens NT1 (34). These two reporter strains have been constructed to produce β-galactosidase in response to the presence of unsubstituted AHLs of C12 to C16 in chain length or substituted AHLs of C4 to C14 in chain length, respectively. A positive response to AHL can be observed as a blue stain on appropriate indicator plates. An AHL produced by E. coli JM109 carrying pAf-act was detected strongly by S. meliloti Rm41 SinI− (dark stain, streak b, Fig. 2A) but only weakly by A. tumefaciens NT1 (barely visible stain, streak b, Fig. 2B). Neither of the reporter strains responded to E. coli JM109 containing plasmid pKK223-3 (lacking act), demonstrating that act is required for the activity (streak a, Fig. 2A and B). As a positive control, both reporter strains respond to S. meliloti Rm41, which produces AHLs of C6 to C16 in chain length (streak c, Fig. 2A and B). This suggests that the act from A. ferrooxidans encodes an enzyme with homoserine lactone synthase function that produces AHLs enriched in chain lengths of C12 to C16 when expressed in the heterologous host E. coli JM109.
FIG. 2.
Demonstration of the biological activity of A. ferrooxidans Act. (A) E. coli containing pAf-act is capable of inducing the expression of β-galactosidase (dark spot in streak b) in a reporter strain of S. meliloti, SinI−. On the other hand, E. coli containing the vector plasmid but lacking act does not express β-galactosidase (streak a). The positive-control S. meliloti Rm41 wild type, which produces AHLs of various chain lengths from C12 to C16 (streak c), induces the expression of β-galactosidase in S. meliloti SinI− (streak c). (B) Same as panel A except that the reporter strain used is A. tumefaciens NT1, which responds mainly to shorter substituted AHLs and AHL chain lengths of C4 to C14.
Identification by GC/MS of the AHL product of Act.
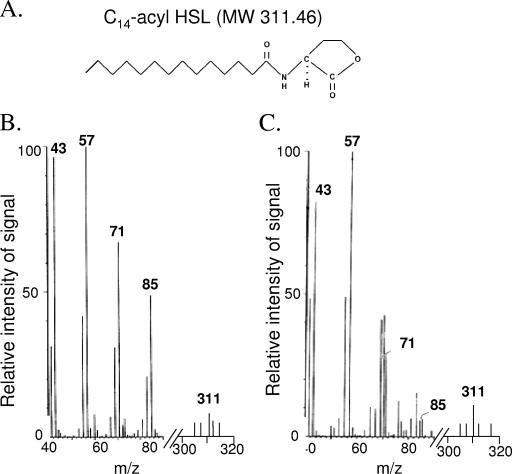
Given the evidence from the bacterial reporter strains for the production of AHLs enriched in chain lengths of C12 to C16 encoded by act, further characterization of the putative AHLs was proposed. E. coli JM109 pAf-act was grown to early stationary phase, and both cells and the supernatant were extracted with DCM. The extract was subjected to preparative fractionation with Sep-Pack C18. Five fractions were recovered (F1 to F5) and independently subjected to GC/MS analysis. The retention times and mass spectra were compared to the retention times and mass spectra from standards of AHL-C14, AHL-C6, and AHL-C8. Fraction F5 contained, as the main product, a compound identified as an unsubstituted AHL of C14 in chain length (Fig. 3); however, the production of minor amounts of other classes of AHLs cannot be discounted. Extracts derived from E. coli JM109 containing the cloning vector pKK223-3 but without act did not yield a product with a mass spectrum related to AHL (data not shown), indicating that the presence of act is required for the production of AHL. It is concluded that Act catalyzes the formation mainly of an unsubstituted AHL of chain length C14.
FIG. 3.
Identification by MS of an AHL in the extracellular supernatant derived from a culture of E. coli JM109 containing pAf-act. (A) Structure of a standard unsubstituted AHL of chain length C14. MW, molecular weight; HSL, homoserine lactone. (B and C) Mass spectra of a supernatant extract from E. coli JM109 pAf-act (B) and a synthetic standard of unsubstituted C14-AHL (C) (the scale to the right of the line break on the x axis has been amplified five times to show the m/z at 311). The characteristic mass-to-ion charge ratios (m/z) of the AHL are indicated.
DISCUSSION
The detection by a reporter strain of bacteria of AHLs produced by the enzyme expressed from a cloned copy of act and the demonstration by GC/MS that these AHLs are enriched in molecules of C14 in chain length provide experimental evidence that Act has AHL synthase activity. Homology-based sequence similarity analyses, including the identification of functional domains and motifs, suggest that Act is related to the LPA acyltransferase family, which includes NlaB from Neisseria meningitidis (35) and PlsC from E. coli (28). In E. coli, the LPA acyltransferase PlsC catalyzes the transfer of an acyl chain from either acyl coenzyme A (acyl-CoA) or acyl-acyl carrier protein onto LPA to produce phosphatidic acid, which, like LPA, is a critical phospholipid intermediate in cell membrane biosynthesis. Act possesses two motifs, KHQSAWET and PEGTR, which are highly conserved in prokaryotic and eukaryotic LPA acyltransferases and which may constitute an acyl-CoA/acyl-acyl carrier protein binding site. This raises the possibility that Act is involved in phospholipid biosynthesis, and it was shown recently that HdtS exhibits LPA acyltransferase activity (EC 2.3.1.51) involved in cell membrane biosynthesis (5). However, amino acid residues outside these motifs may alter the activity or specificity of these acyltransferases (35, 42) and could allow the transfer of an acyl group from acetyl-CoA to a substrate such as S-adenosylmethionine to form AHL as found in the classical LuxI system.
The experimental evidence that HdtS is involved in both the acylation of LPA in cell membrane biosynthesis (5) and the acylation of S-adenosylmethionine in AHL biosynthesis (reference 15 and this paper) suggests that it has a dual function. Coordinated expression of cell membrane biosynthesis and the production of AHLs could be a means for cells to communicate that cell division is taking place and that cell density increases could be occurring. A relationship has also been established between cell membrane fluidity changes due to phospholipid decreases resulting from stress and the activation of AHLs and QS (2).
Bioinformatic prediction and experimental validation demonstrate that act is found in a transcriptional unit together with the predicted genes glyQ, glyS, and gph predicted to encode glycyl-tRNA synthetase α chain (EC 6.1.1.14), glycyl-tRNA synthetase β chain (EC 6.1.1.14), and a phosphatase (general function), respectively. This locus appears to be highly conserved, including the gene order, in a wide range of proteobacteria (data not shown).
The role of the predicted phosphatase in this locus is not known. However, it has been shown that in eukaryotic systems a phosphatidate phosphatase (EC 2.3.1.15) carries out the conversion of 1,2-diacyl-sn-glycerol 3-phosphate (one of the potential products of Act) to 1,2-diacyl-sn-glycerol, which is subsequently used for phospholipid biosynthesis (43). It is possible that the predicted phosphatase encoded by gph assumes this role in bacteria, even though it exhibits no sequence similarity to its eukaryotic counterpart.
The conservation of glyQS in the act locus is more enigmatic. In addition to its role in phospholipid metabolism, 1,2-diacyl-sn-glycerol feeds into a pathway for the biosynthesis of glycine which may connect it with the requirement for coordinated expression of glycine tRNA synthetase. An alternative hypothesis is that glycine tRNA synthetase can act as a glycine donor in the cross-linking of peptidoglycan via a characteristic pentaglycine interpeptide bridge, as has been demonstrated in the gram-positive bacteria Staphylococcus, Weissella, Streptococcus, Renibacterium, and Bifidobacterium spp. and the gram-negative bacteria Treponema and Borrelia spp. (31). The coordinated formation of peptidoglycan and cell membrane constituents during cell division and cell membrane and cell wall repair has been established (9, 37), potentially requiring the coordinated expression of glycine tRNA synthetase and phospholipid biosynthesis.
act is more highly expressed when cells are grown in iron-containing medium than when they are grown in sulfur-containing medium, which is the converse of what has been observed for the LuxIR QS genes of A. ferrooxidans (8, 27). This demonstrates that A. ferrooxidans has two distinct AHL-based mechanisms for QS. The function of these two systems may depend upon environmental signals that are related to the ability of A. ferrooxidans to colonize and use different solid sulfur- and iron-containing minerals. Both QS systems mediate their effects through the biosynthesis of AHLs. Intercellular communication signals in the environment inhabited by A. ferrooxidans would need to be acid stable, and AHLs produced by A. ferrooxidans have been shown to be active in acid medium (8, 27). The stability of other QS signals in extremely acidic conditions is unknown.
Although a candidate promoter for the act operon was detected, a search for known transcription factor binding sites was not successful; therefore, details as to the mechanism(s) that differentially regulates the expression of act and luxI await further study. Other organisms are known to produce and respond to more than one QS signal. For example, P. aeruginosa produces two AHLs that bind and activate respectively LasR and RlhR transcription factors, both of which are members of the LuxR family of helix-turn-helix transcription factors (23). Vibrio harveyi produces and responds to three autoinducers, and this sensory information converges to control the expression of bioluminescence, biofilm formation, type III secretion, and protease production (41).
The physiological responses controlled by the putative Act QS system of A. ferrooxidans remain to be investigated. In other bacteria QS has been shown to be involved in production of antibiotics and virulence factors, conjugation, transformation, swarming behavior, and biofilm formation (20); coaggregation (11); and the production of biosurfactants (6). It has been suggested that biofilm formation in A. ferrooxidans might be under the control of the LuxIR system (8, 27). Given that A. ferrooxidans forms biofilms on different iron- and sulfur-containing mineral surfaces such as pyrite (FeS2) and chalcocite (Cu2S) (24, 30), perhaps via extracellular polysaccharide formation (1), the possible roles of the LuxIR and Act systems in biofilm formation on different minerals become an attractive option, especially as they could help to explain the opposite responses of the LuxIR and ActS systems to iron and sulfur, respectively. This suggests the presence of signaling cascades, and future work will be directed towards the discovery of gene targets of the LuxIR and Act systems and how they might be integrated to produce appropriate physiological responses dictated by environmental signals.
Acknowledgments
The work was supported by Fondecyt grant no. 1050063 and a Microsoft Sponsored Research Award. M.S. thanks the Millennium Scientific Initiative through Millennium Nucleus EMBA grant P04/007-F and USM grant 130522 for financial support.
We thank Stephen Winans for providing cultures of Agrobacterium tumefaciens NT1 and Juan Gonzalez for providing Sinorhizobium meliloti Rm41 and Rm41 SinI−. We thank Myriam González from Universidad Técnica Federico Santa María, Valparaíso, for assistance with the GC/MS experiments.
Footnotes
Published ahead of print on 9 March 2007.
REFERENCES
- 1.Barreto, M., E. Jedlicki, and D. S. Holmes. 2005. A gene cluster for the formation of extracellular polysaccharide precursors in the chemolithoautotroph Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 71:2907-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baysse, C., M. Cullinane, V. Denervaud, E. Burrowes, J. M. Dow, J. P. Morrisey, L. Ram, J. T. Trevors, and F. O'Gara. 2005. Modulation of quorum sensing in Pseudomonas aeruginosa through alteration of membrane properties. Microbiology 151:2529-2542. [DOI] [PubMed] [Google Scholar]
- 3.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughsonet. 2002. Structural identification of a bacterial quorum sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 4.Crooks, G. E., G. Hon, J. M. Chandonia, and S. E. Brenner. 2004. WebLogo: a sequence logo generator. Genome Res. 14:1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullinane, M., C. Baysse, J. P. Morrissey, and F. O'Gara. 2005. Identification of two lysophosphatidic acid acyltransferase genes with overlapping function in Pseudomonas fluorescens. Microbiology 151:3071-3080. [DOI] [PubMed] [Google Scholar]
- 6.Daniels, R., S. Reynaert, S. H. Hoekstra, C. Verreth, J. Janssens, K. Braeken, M. Fauvart, S. Beullens, C. Heusdens, I. Lambrichts, D. E. De Vos, J. Vanderleyden, J. Vermant, and J. J. Michiels. 2006. Quorum signal molecules as biosurfactants affecting swarming in Rhizobium etli. Proc. Natl. Acad. Sci. USA 103:14965-14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunny, G. M., and B. A. Leonard. 1997. Cell-cell communication in gram-positive bacteria. Annu. Rev. Microbiol. 51:527-564. [DOI] [PubMed] [Google Scholar]
- 8.Farah, C., M. Vera, D. Morin, D. Haras, C. A. Jerez, and N. Guiliani. 2005. Evidence for a functional quorum-sensing type AI-1 system in the extremophile bacterium Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 71:7033-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman, R. C., and A. Branstrom. 1999. Targeting cell wall synthesis and assembly in microbes: similarities and contrast between bacteria and fungi. Curr. Pharm. Des. 5:473-501. [PubMed] [Google Scholar]
- 10.Guacucano, M., G. Levican, D. S. Holmes, and E. Jedlicki. 2000. An RT-PCR artifact in the characterization of bacterial operons. Electron. J. Biotechnol. 3:213-216. [Google Scholar]
- 11.Jiang, H. L., J. H. Tay, A. M. Maszenan, and S. T. Tay. 2006. Enhanced phenol biodegradation and aerobic granulation by two coaggregating bacterial strains. Environ. Sci. Technol. 40:6137-6142. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, D. B. 1998. Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol. Ecol. 27:307-317. [Google Scholar]
- 13.Johnson, M. R., C. I. Montero, S. B. Conners, K. R. Shockley, S. L. Bridger, and R. M. Kelly. 2005. Population density-dependent regulation of exopolysaccharide formation in the hyperthermophilic bacterium Thermotoga maritima. Mol. Microbiol. 55:664-674. [DOI] [PubMed] [Google Scholar]
- 14.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. A. B. Stewart, A. Lazduski, and P. Williams. 1995. Multiple homologues of Lux R and Lux I control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 15.Laue, B. E., Y. Jiang, S. R. Chhabra, S. Jacob, G. S. A. B. Stewart, A. Hardman, J. A. Downie, F. O'Gara, and P. Williams. 2000. The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N-(3-hydroxy-7-cis-tetradecenoyl) homoserine lactone, via HdtS, a putative novel N-acylhomoserine lactone synthase. Microbiology 146:2469-2480. [DOI] [PubMed] [Google Scholar]
- 16.Liu, Z., N. Guiliani, C. Appya-Ayme, F. Borne, J. Ratouchniak, and V. Bonnefoy. 2000. Construction and characterization of a recA mutant of Thiobacillus ferrooxidans by marker exchange mutagenesis. J. Bacteriol. 182:2269-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llamas, I., E. Quesada, M. J. Martinez-Canovas, M. Gronquist, A. Eberhard, and J. E. Gonzalez. 2005. Quorum sensing in halophilic bacteria: detection of N-acyl-homoserine lactones in the exopolysaccharide-producing species of Halomonas. Extremophiles 9:333-341. [DOI] [PubMed] [Google Scholar]
- 18.Llamas, I., N. Keshavan, and J. E. Gonzalez. 2004. Use of Sinorhizobium meliloti as an indicator for specific detection of long-chain N-acyl homoserine lactones. Appl. Environ. Microbiol. 70:3715-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClean, K., M. Winson, L. Fish, A. Taylor, S. Chhabra, M. Camara, M. Daykin, J. Lamb, S. Swift, B. Bycroft, G. S. A. B. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 20.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 21.Paggi, R. A., C. B. Martone, C. Fuqua, and R. E. De Castro. 2003. Detection of quorum sensing signals in the haloalkaliphilic archaeon Natronococcus occultus. FEMS Microbiol. Lett. 221:49-52. [DOI] [PubMed] [Google Scholar]
- 22.Pappas, K. M., C. L. Weingart, and S. C. Winans. 2004. Chemical communication in proteobacteria: biochemical and structural studies of signal synthases and receptors required for intercellular signaling. Mol. Microbiol. 53:755-769. [DOI] [PubMed] [Google Scholar]
- 23.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawlings, D. E. 2002. Heavy metal mining using microbes. Annu. Rev. Microbiol. 56:65-91. [DOI] [PubMed] [Google Scholar]
- 25.Rawlings, D. E. 2005. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Factories 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reading, N. C., and V. Sperandio. 2006. Quorum sensing: the many languages of bacteria. FEMS Microbiol. Lett. 254:1-11. [DOI] [PubMed] [Google Scholar]
- 27.Rivas, M., M. Seeger, D. S. Holmes, and E. Jedlicki. 2005. A Lux-like quorum sensing system in the extreme acidophile Acidithiobacillus ferrooxidans. Biol. Res. 38:283-297. [DOI] [PubMed] [Google Scholar]
- 28.Rock, C., S. Jackowski, and J. Cronan. 1996. Lipid metabolism in prokaryotes, p. 35-74. In D. E. Vance and J. E. Vance (ed.), Biochemistry of lipids, lipoproteins and membranes. Elsevier, Amsterdam, The Netherlands.
- 29.Sambrook, J. E., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Schippers, A., and W. Sand. 1999. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 65:319-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider, T., M. M. Senn, B. Berger-Bachi, A. Tossi, H. G. Sahl, and I. Wiedemann. 2004. In vitro assembly of a complete, pentaglycine interpeptide bridge containing cell wall precursor (lipid II-Gly5) of Staphylococcus aureus. Mol. Microbiol. 53:675-685. [DOI] [PubMed] [Google Scholar]
- 32.Seeger, M., B. Cámara, and B. Hofer. 2001. Dehalogenation, denitration, dehydroxylation, and angular attack of substituted biphenyls and related compounds by a biphenyl dioxygenase. J. Bacteriol. 183:3548-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeger, M., M. González, B. Cámara, L. Muñoz, E. Ponce, L. Mejías, C. Mascayano, Y. Vásquez, and S. Sepúlveda-Boza. 2003. Biotransformation of natural and synthetic isoflavonoids by two recombinant microbial enzymes. Appl. Environ. Microbiol. 69:5045-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw, P., G. Ping, S. Daly, C. Cha, J. Cronan, K. Rinehart, and K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shih, G., C. Kahler, J. Swartley, M. Rahman, J. Coleman, R. Carlson, and D. Stevens. 1999. Multiple lysophosphatidic acid transferases in Neisseria meningitidis. Mol. Microbiol. 32:942-952. [DOI] [PubMed] [Google Scholar]
- 36.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart, G. C. 2005. Taking shape: control of bacterial cell wall biosynthesis. Mol. Microbiol. 57:1177-1181. [DOI] [PubMed] [Google Scholar]
- 38.Sun, J., R. Daniel, I. Wagner-Döbler, and A. P. Zeng. 2004. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol. Biol. 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swift, S., A. Karlyshev, L. Fish, E. Durant, and M. Winson. 1997. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acyl homoserine lactone signal molecules. J. Bacteriol. 179:5271-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swift, S., P. Williams, and G. S. A. B. Stewart. 1999. N-Acylhomoserine lactones and quorum sensing in proteobacteria, p. 291-313. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, DC.
- 41.Waters, C. M., and B. L. Bassler. 2006. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev. 20:2754-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West, J., C. K. Tompkins, N. Balantac, E. Nudelman, B. Meengs, and T. White. 1997. Cloning and expression of two human lysophosphatidic acid acyltransferase cDNAs that encode cytokine-induced signaling response in cells. DNA Cell Biol. 16:691-701. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita, S., K. Hosaka, and S. Numa. 1973. Acyl-donor specificities of partially purified 1-acylglycerophosphate acyltransferase, 2-acylglycerophosphate acyltransferase and 1-acylglycerophosphorylcholine acyltransferase from rat-liver microsomes. Eur. J. Biochem. 38:25-31. [DOI] [PubMed] [Google Scholar]
- 44.Yates, J., and D. S. Holmes. 1988. Two families of repeated DNA sequences in Thiobacillus ferrooxidans. Proc. Natl. Acad. Sci. USA 85:7284-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]