Abstract
Recently we showed that degradation of several nonylphenol isomers with α-quaternary carbon atoms is initiated by ipso-hydroxylation in Sphingobium xenophagum Bayram (F. L. P. Gabriel, A. Heidlberger, D. Rentsch, W. Giger, K. Guenther, and H.-P. E. Kohler, J. Biol. Chem. 280:15526-15533, 2005). Here, we demonstrate with 18O-labeling experiments that the ipso-hydroxy group was derived from molecular oxygen and that, in the major pathway for cleavage of the alkyl moiety, the resulting nonanol metabolite contained an oxygen atom originating from water and not from the ipso-hydroxy group, as was previously assumed. Our results clearly show that the alkyl cation derived from the α-quaternary nonylphenol 4-(1-ethyl-1,4-dimethyl-pentyl)-phenol through ipso-hydroxylation and subsequent dissociation of the 4-alkyl-4-hydroxy-cyclohexadienone intermediate preferentially combines with a molecule of water to yield the corresponding alcohol and hydroquinone. However, the metabolism of certain α,α-dimethyl-substituted nonylphenols appears to also involve a reaction of the cation with the ipso-hydroxy group to form the corresponding 4-alkoxyphenols. Growth, oxygen uptake, and 18O-labeling experiments clearly indicate that strain Bayram metabolized 4-t-butoxyphenol by ipso-hydroxylation to a hemiketal followed by spontaneous dissociation to the corresponding alcohol and p-quinone. Hydroquinone effected high oxygen uptake in assays with induced resting cells as well as in assays with cell extracts. This further corroborates the role of hydroquinone as the ring cleavage intermediate during degradation of 4-nonylphenols and 4-alkoxyphenols.
Technical nonylphenol is a complex mixture of more than 100 isomers which differ in the structure and the position of the alkyl moiety attached to the phenol ring (20). More than 90% of the mixture consists of para-substituted nonylphenols (36, 40). The technical product serves mainly in the manufacture of nonylphenol polyethoxylates, a class of nonionic surfactants that have a wide range of industrial applications and are used in large amounts worldwide (36). Because such surfactants are designed for usage in aqueous solutions, they are discharged mainly into wastewaters and thereby enter sewage treatment plants, where they are rapidly degraded to more-recalcitrant metabolites, such as short-chain nonylphenol ethoxylates, carboxylic acid derivatives, and nonylphenols (1, 2, 4, 38). Nonylphenols are highly toxic to aquatic organisms (25, 32, 35) and are able to mimic estrogens in fishes, mammals, and other animals (33, 35, 41). The estrogenic activity of the individual isomers varies widely (16, 23), and therefore the isomeric composition of nonylphenol mixtures needs to be taken into account for investigations about the environmental fate and the toxic effects of nonylphenol compounds (17).
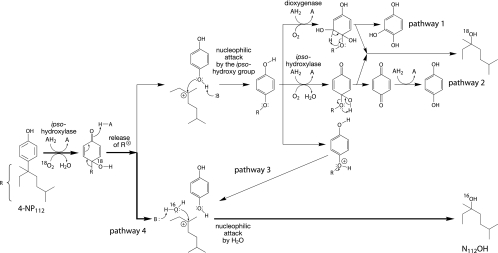
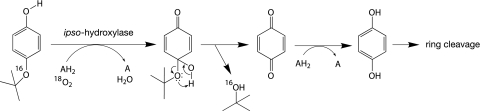
Recently isolated microorganisms (12, 13, 37) are able to grow with α-quaternary nonylphenols as the sole sources of carbon and energy. These bacteria degrade nonylphenols through similar pathways; they release the nonyl substituent as nonanol with unchanged carbon connectivity into the culture medium and most likely utilize only the ring moiety as an energy and carbon source. The conversion of the alkyl chain into the corresponding α-alcohol was explained by an ipso-substitution mechanism in which the nonyl substituent is replaced as a ring-bonding partner by the oxygen atom of the oxidizing species (6, 14, 30). According to this mechanism, the nonylphenol degradation pathway starts with an ipso-hydroxylation, which yields a 4-hydroxy-4-nonyl-cyclohexadienone intermediate (quinol), from which an α-quaternary alkyl chain can be detached as a short-living cation (Fig. 1). Alkyl branches attached to the carbocation help to delocalize and thereby stabilize the positive charge through inductive and hyperconjugative effects, which explains why only alkyl moieties of α-quaternary nonylphenols are released (13, 14). This view was corroborated by experiments with Sphingobium xenophagum Bayram (formerly Sphingomonas xenophaga Bayram [31]), in which the alkyl chains of the non-α-quaternary isomers 4-(1-methyl-octyl)-phenol (4-NP2) and 4-n-nonylphenol (4-NP1) (Fig. 2) were not released, so the bacterium was unable to utilize these isomers as growth substrates (13, 14). It was proposed that the carbocation quickly reacts with a nucleophilic oxygen atom, presumably with that of the deprotonated geminal ipso-hydroxy group (Fig. 1, pathways 1 to 3) (14), or alternatively, with that of a molecule of water (Fig. 1, pathway 4) (6, 30, 39). The reaction with the ipso-oxygen atom would yield a 4-alkoxyphenol intermediate, from which the side chain can be released as a nonanol by known mechanisms (Fig. 1, pathways 1 and 2) (11, 18).
FIG. 1.
Potential pathways for the degradation of α-quaternary 4-nonylphenols in Sphingobium xenophagum Bayram. Nonylphenols are transformed by an initial ipso-hydroxylation to 4-alkyl-4-hydroxy-cyclohexadienone intermediates. α-Quaternary intermediates dissociate by releasing the alkyl moiety as a cation, which is stabilized by α-alkyl branching (14). Degradation experiments with 18O-labeled oxygen and water clearly show that the detached cation preferentially reacts with the nucleophilic oxygen atom of a water molecule (pathway 4). However, the degradation of a small fraction (up to 13%) of certain α,α-dimethyl-substituted nonylphenols appears to proceed on a minor pathway, in which the cation reacts with the ipso-hydroxy group (pathway 2, or possibly pathway 1). Pathway 3 was ruled out by showing that the ether oxygen in 4-t-butoxyphenol, a structural analogue of α-quaternary 4-nonyloxyphenols, was retained in the t-butanol metabolite. To show incorporation of molecular oxygen during the ipso-hydroxylation step, 4-(1-methyl-octyl)-phenol, which contains an α-hydrogen atom and is converted to accumulating ipso-hydroxylated metabolites, was used as a substrate analogue (13, 14). AH2 and A, a pair of reduced and oxidized cosubstrates, respectively; B, a proton acceptor.
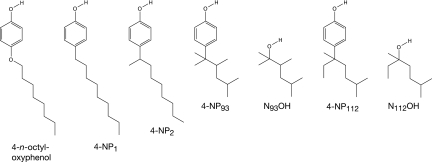
FIG. 2.
Structures of 4-n-octyloxyphenol and of the 4-nonylphenol and nonanol isomers mentioned in this study. 4-NP1, 4-n-nonylphenol; 4-NP2, 4-(1-methyl-octyl)-phenol; 4-NP93, 4-(1,1,2,4-tetramethyl-pentyl)-phenol; N93OH, 2,3,5-trimethyl-hexan-2-ol; 4-NP112, 4-(1-ethyl-1,4-dimethyl-pentyl)-phenol; N112OH, 3,6-dimethyl-heptan-3-ol.
To elucidate the exact mechanism for the cleavage of the nonyl moiety during degradation of α-quaternary nonylphenols by strain Bayram, we have conducted 18O-labeling experiments. Our results clearly show that the ipso-hydroxy group originates from molecular oxygen and that the oxygen atom incorporated into the nonanol metabolite derives mainly from water and not, as previously assumed, from the ipso-hydroxy function (14). Furthermore, we clearly established that strain Bayram is indeed capable of degrading alkoxyphenols by ipso-substitution, via a hemiketal derivative.
MATERIALS AND METHODS
Chemicals.
The acronyms for the various 4-nonylphenol isomers used in this study are based on the systematic numbering systems proposed by Guenther and coworkers (17). The sources and purities of the nonylphenol isomers 4-NP1, 4-NP2, 4-(1,1,2,4-tetramethyl-pentyl)-phenol (4-NP93), and 4-(1-ethyl-1,4-dimethyl-pentyl)-phenol (4-NP112) (Fig. 2) have been described elsewhere (13). 4-t-Butoxyphenol (98%) and 4-n-octyloxyphenol (98%) (Fig. 2) were purchased from Matrix Scientific (Columbia, SC) and Acros Organics (Morris Plains, NJ), respectively. N,O-Bis-(trimethylsilyl)-trifluoracetamide (BSTFA; 98%) was obtained from Fluka (Buchs, Switzerland).
Growth of cells for oxygen uptake experiments.
Cells were grown in a 20-liter vessel (diameter, 28 cm; height, 33 cm; closed with a silicone stopper) containing 16 liters of medium that consisted of 1/10 diluted LB medium (13), 2.1 g CaCl2·2H2O (0.9 mM), 16.4 g MgSO4·7H2O (4.2 mM), 80.0 g (NH4)2SO4 (37.8 mM), and 16.0 g KH2PO4 (7.4 mM). The LB and PO4 components were autoclaved separately, and their pH values were adjusted to 7.0 with NaOH. An aqueous vitamin solution (16 ml) containing (+)-biotin (20 μg/ml), folic acid (20 μg/ml), pyridoxine (100 μg/ml), thiamine hydrochloride (50 μg/ml), riboflavin (50 μg/ml), nicotinic acid (50 μg/ml), vitamin B12 (50 μg/ml), calcium d-pantothenate (50 μg/ml), 4-aminobenzoic acid (50 μg/ml), dl-α-lipoic acid (50 μg/ml), and nicotinamide (50 μg/ml) and an aqueous solution of trace elements (16 ml) made up of 32% HCl (10 μl/ml), FeSO4·7H2O (2,100 μg/ml), H3BO3 (60 μg/ml), MnCl2·4H2O (100 μg/ml), CoCl2·6H2O (120 μg/ml), NiCl2·6H2O (25 μg/ml), CuCl2·2H2O (15 μg/ml), ZnCl2 (70 μg/ml), Na2MoO4·2H2O (25 μg/ml), and EDTA-Na4·4H2O (7,000 μg/ml) (the pH was adjusted to a value of 6.0 with NaOH) were added by sterile filtration. The medium was inoculated with about 180 ml of a liquid culture of strain Bayram (optical density ≈ 0.25) that was grown for 2 days in minimal medium with 1 mg/ml technical nonylphenol as the sole carbon and energy source. To induce the cells, technical nonylphenol (16 ml) was added to the 16-liter culture by means of a needle-mounted sterile syringe. During incubation, the medium was aerated with a cylindrical air stone (Oxygenius Micro-Ceramic L, 150 mm; Europet, The Netherlands), and it was stirred with a magnetic Teflon bar (diameter, 2.5 cm; length, 10 cm). Care was taken that the undissolved viscous nonylphenol remained on the surface of the culture medium.
Preparation of washed cell suspensions and cell extract.
Cells of the 16-liter culture were harvested after 48 h of incubation by centrifugation (10,000 × g, 4°C, 10 min). At this time, the optical density at 546 nm had reached a value of about 1.4. After being washed with an excess of 20 mM Na phosphate buffer (pH 7.4), bacteria were resuspended in the same buffer (cells were concentrated 10 times to approximately 4.8 mg [dry weight] per ml of buffer). To determine the dry weight per volume, an aliquot of the suspension was filtered through a preweighed filter (Nuclepore polycarbonate; Costar, Cambridge, MA) with a pore size of 0.2 μm and a diameter of 25 mm, and the deposit was lyophilized until a constant weight was reached. To prepare the crude cell extracts, cells were suspended in the phosphate buffer described above and disrupted by ultrasonication. Cell fragments were separated by centrifugation for 10 min at 16,000 × g and 4°C.
Oxygen uptake experiments with resting cells.
Oxygen uptake rates were measured polarigraphically with an O2 electrode (voltage set at 0.6 V) mounted on a temperature-controlled reaction vessel (2.5 ml, 23 to 25°C) equipped with a magnetic stirrer (digital model 10 controller; Rank Brothers, Ltd., Cambridge, United Kingdom). For monitoring, LabVIEW 7.0 Express (National Instruments, Austin, TX; program development by Eawag Informatics Support [P. Perisset]) was run on a Power Macintosh G3, with a 6034E data acquisition board (E series; National Instruments) as the interface. Before each experiment, the reaction vessel and the chamber piston were rinsed successively with H2O (twice), CH3OH (once), H2O (twice), and 20 mM Na phosphate buffer, pH 7.4 (twice). The assay mixture contained 2.4 ml of 20 mM Na phosphate buffer, pH 7.4, and 0.3 ml of the washed cell suspension. The excess volume was displaced through the injection channel in the chamber piston. The reactions were started by adding substrates as a concentrated methanol solution (0.31 mM in the reaction mixture). Methanol in the concentration range applied did not have any effect on the assay. Resting cells were kept at 4°C and were utilized within 48 h. During this time period, the cells remained fully active.
Growth experiments.
Degradation experiments with strain Bayram and minimal medium (3 ml yeast nitrogen base without amino acid; Difco, Detroit, MI) containing 4-t-butoxyphenol and 4-n-octyloxyphenol (1 mg/ml) (Fig. 2) as the sole sources of carbon and energy were set up as described previously (13). Substrate concentrations at various time points were determined by extracting the cultures with CH2Cl2 and analyzing the extracts with high-pressure liquid chromatography-UV (13).
Isotope-labeling experiments.
Experiments with 18O2-enriched air were carried out in 25-ml (or 13-ml) crimp-sealed serum flasks. For experiments with H218O-enriched media, we utilized screw-cap cylindrical vials with a ratio of air space to culture volume of at least 13. In the degradation experiment with 4-NP112 and 300 μl medium (95% H218O) (see Fig. 5B), the 4-ml vial was only loosely closed to ameliorate the aeration of the medium. Generally, phenolic substrates (0.7 to 1.0 mg per ml medium) were added to autoclaved, empty culture vials and dissolved in hexane (nonylphenols) or acetone (alkoxyphenols), and the solvent was evaporated in a sterile laminar-flow bench. Sterile minimal medium (1.0 ml) was then added. The cometabolic transformation of 4-NP2 (0.5 mg) was studied with 4-NP112 (0.5 mg) as the sole source of carbon and energy. To set up experiments with labeled water, the phenolic substrate was added to 300 μl of lyophilized medium, and 300 μl of labeled water (95% H218O, 5% H216O; Cambridge Isotope Laboratories, Andover, MA) was added. Culture media with 19% H218O were prepared by adding 300 μl labeled water to 1.2 ml minimal medium. To verify that an exchange of the hydroxy function of the nonanol metabolites for H2O did not occur, we performed a degradation experiment with 1 mg/ml 4-NP93 and 19% H218O and added 3,6-dimethyl-heptan-3-ol (N112OH) (Fig. 2) when the culture had become obviously turbid. All media were inoculated with 100 μl of a liquid culture or with colonies of strain Bayram grown on minimal medium with technical nonylphenol as the sole source of carbon and energy. For labeling experiments using 18O2, the headspace in each sealed serum flask was flushed for 6 min with sterile filtered N2. A 10-ml volume of isotope-labeled O2 (Euriso-Top, Gif-sur-Yvette, France), which had an 18O2/16O2 ratio of 95.6/4.4 as determined by mass spectrometry (MS), was then injected into the vials. Regular culture vials and appropriate controls (uninoculated, unlabeled) were incubated at 25°C on a shaker (240 rpm). Cultures were analyzed when they became obviously turbid.
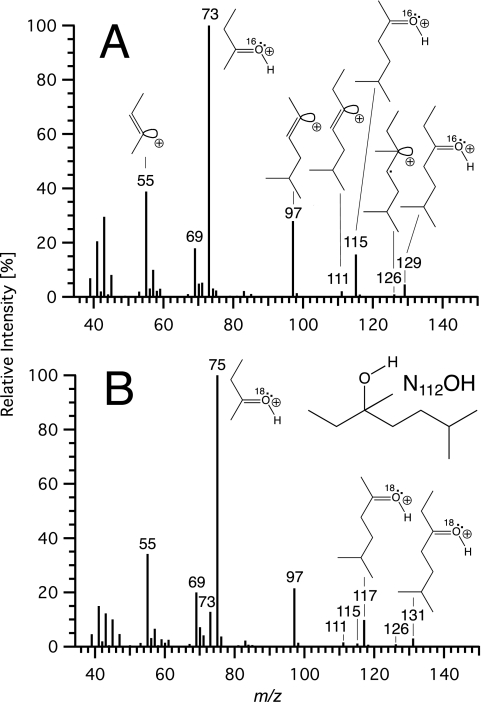
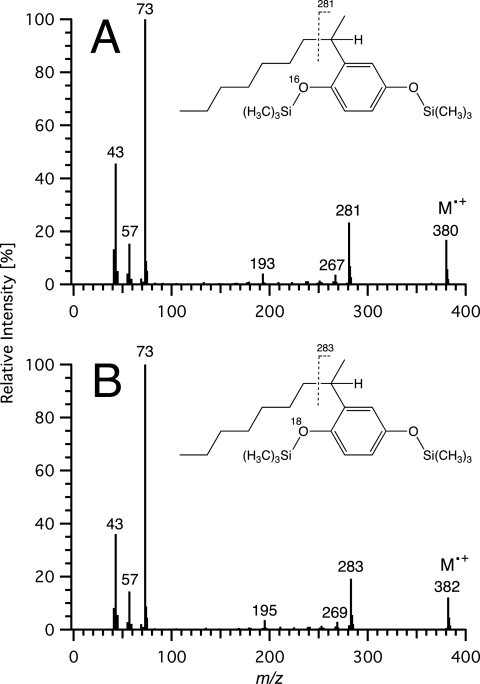
FIG. 5.
Origin of the oxygen atom of the nonanol derived from 4-(1-ethyl-1,4-dimethyl-pentyl)-phenol (4-NP112). EI mass spectra of 3,6-dimethylheptan-3-ol (N112OH) formed by strain Bayram from 4-NP112 in an atmosphere enriched with 18O2 (18O2/16O2 ratio ≈ 95.6/4.4) (A) and in a medium containing 95% H218O (5% H216O) (B).
Analytical procedures.
Metabolites were analyzed with a GC 8060 gas chromatograph (Fisons Instruments, Milan, Italy) coupled to an MD 800 single-quadrupole MS (Fisons Instruments, Manchester, United Kingdom). Interface and source temperatures were set at 250 and 200°C, respectively. Substances were ionized with electron impact (EI; 70 eV, if not otherwise specified) and data acquired in the full-scan mode. For the analysis of nonanols, 2 μl of an appropriately diluted CH2Cl2 extract of the culture was introduced into a splitless injector heated to 270°C. The separation of the analytes was achieved on a 60-m DB-17 MS capillary column (2 by 30 m, 0.25-mm internal diameter, 0.25-μm film thickness; J&W Scientific, Folsom, CA), linked to a 2-m guard and a 2-m transfer column (0.32-mm and 0.18-mm internal diameters, respectively; deactivated with OV-1701-OH; BGB Analytic Ag, Böckten, Switzerland). Helium was used as the carrier gas at a constant flow of 1.2 ml/min. The following temperature program was applied: increases from 50°C to 220°C at 3°C/min, and then 5 min at 220°C. To achieve an optimal separation of the nonanols formed in the degradation experiment with technical nonylphenol, an appropriately diluted ethyl acetate extract of the culture was injected on-column into a 60-m Stabilwax separation column (see below). The helium carrier gas flow was held constant at 1.2 ml/min. We applied the following temperature program: 0.5 min at 50°C, increases to 180°C at 3°C/min, and then 5 min at 180°C.
The products of the cometabolic transformation of 4-NP2 were derivatized with trimethylsilyl groups prior to gas chromatography (GC) analysis. For this purpose, the CH2Cl2 extract of the culture was dried under a stream of N2 and repeatedly dissolved with a volume of 200 μl CH3CN to eliminate traces of water. The residue was then incubated overnight at room temperature with 200 μl of BSTFA, dried under a stream of N2, and washed twice with 200 μl of CH3CN. Two microliters of an appropriately diluted CH2Cl2 solution were introduced into the splitless injector. The GC-MS analysis was done according to the analysis of nonanols with the DB-17 MS capillary column, but with 45 eV electron energy and the following temperature program: increases from 60°C to 240°C at 10°C/min and then 10 min at 240°C.
The analysis of the t-butanol metabolite was performed by direct-aqueous-injection GC-MS (43). The sterile filtrate (0.2 μm) of a diluted culture aliquot was introduced manually through a cold on-column injector into a 10-m by 0.53-mm deactivated guard column (OV-1701-OH). Separation was achieved on a 60-m by 0.32-mm Stabilwax fused-silica column coated with a 1.0-μm cross-bonded Carbowax-poly(ethylene glycol) film (Restek, Bellefonte, PA). A 2-m by 0.18-mm column (OV-1701-OH) ensured the transfer to the mass spectrometer. The helium carrier gas flow was held constant at 1.4 ml/min. We applied the following temperature program (modified according to reference 43): 0.5-min standby at 60°C, 7.5 min at 60°C, increased to 90°C at 12°C/min, 5 min at 90°C, increased to 200°C at 30°C/min, 6 min at 200°C, and 6-min postrun time. The 7.5-min-long plateau at 60°C, the bake time at 200°C, and the long postrun time were required for the complete elimination of water.
To determine the actual composition of 18O-labeled O2 in the gas bottle, samples were analyzed by means of a sector field mass analyzer in EI mode (MAT95; Finnigan, San Jose, CA) with an ionization energy of 70 eV.
RESULTS
Oxygen uptake experiments.
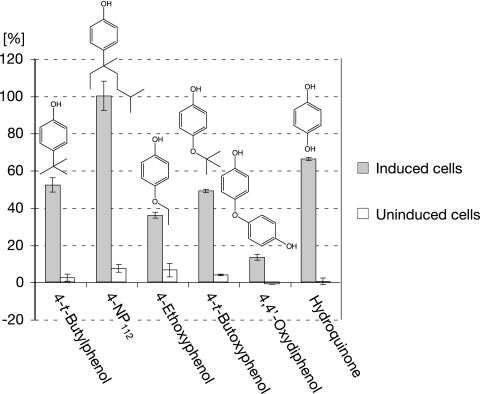
In order to elucidate the substrate preference of the ipso-hydroxylating activity, a series of para-substituted phenolic compounds was tested for effecting oxygen uptake in resting-cell assays. Oxygen consumption of resting cells of strain Bayram grown in a medium enriched with tryptone and yeast extract and induced with technical nonylphenol was greatly enhanced by the addition of 4-NP112; this compound, which is present in major amounts in the technical mixture, showed the highest activity among the series of test compounds (Fig. 3). Moreover, 4-ethoxyphenol, 4-t-butoxyphenol, and 4,4′-oxydiphenol clearly enhanced O2 uptake by induced cells. On the other hand, 4-n-octyloxyphenol (Fig. 2) and 4-methoxyphenol, as well as 4-NP1 and 4-alkylphenols with fewer than four carbon atoms in the alkyl chain, did not effect enhanced oxygen consumption (Fig. 3, legend). In control experiments, noninduced cells grown in the absence of nonylphenol did not respond to any of the substrates tested. This indicates that the hydroxylating enzyme activities responsible for initiating degradation were inducible and that the degradation pathways of 4-alkyl- and 4-alkoxyphenols might coincide. It is noteworthy that the oxygen uptake activity of a test compound, its ability to sustain growth, and its suitability as an ipso-hydroxylation substrate did not correlate in some cases. In particular, the nongrowth substrate 4-t-butylphenol showed approximately the same intensive oxygen uptake activity (50%) as the growth substrate 4-t-butoxyphenol (Fig. 3). Moreover, the lack of activity of the cometabolically transformable 4-NP1 (13, 14) and of the growth substrate 4-n-octylphenol indicated that the rate of ipso-hydroxylation of these substrates was too low to be measurable.
FIG. 3.
Oxygen uptake rates with various compounds by resting-cell suspensions of S. xenophagum grown in the presence and the absence of technical nonylphenol. Specific oxygen uptake rates were corrected for endogenous oxygen uptake (3.1 to 8.9 nmol O2 × min−1 × mg cells [dry weight]−1) and expressed as percentages of the specific rate of O2 consumption of the induced cells caused by 4-NP112 (65.4 nmol O2 × min−1 × mg cells [dry weight]−1). The data points represent the mean values of at least two measurements, and the corresponding standard deviations are expressed with error bars. The following substrates had an activity lower than 4% and were not included in the figure: p-cresol, 4-ethylphenol, 4-n-propylphenol, 4-isopropylphenol, 4-n-nonylphenol, 4-hydroxybenzoic acid, 4-hydroxybenzonitrile, 4-methoxyphenol, 4-n-octyloxyphenol, 4-fluorophenol, 4-chlorophenol, 4-nitrophenol, catechol, resorcinol, and p-benzoquinone.
In contrast to catechol, which had no effect, hydroquinone markedly enhanced the oxygen uptake of induced resting cells (Fig. 3), and such activity was also observed with cell extracts without the addition of reduced cofactors. This is a strong indication that hydroquinone was the ring cleavage intermediate during degradation of nonylphenol.
Growth with alkoxyphenols.
Growth experiments with strain Bayram revealed that the two alkoxyphenols 4-t-butoxyphenol and 4-n-octyloxyphenol promoted growth and were completely transformed within about 2 weeks. Analysis of 4-t-butoxyphenol-grown cultures of strain Bayram by means of GC unequivocally demonstrated the presence of a metabolite with a retention time and a mass spectrum identical to those of authentic t-butanol.
Labeling experiments with 18O-enriched O2 and H2O.
With regard to the degradation of nonylphenols, the oxygen atom incorporated into the nonanol metabolite may originate from either molecular oxygen (Fig. 1, pathways 1 and 2) or a water molecule (pathways 3 and 4), depending on the hydroxylation mechanism and the type of nucleophile reacting with the nonyl cation. In a first step, we demonstrated that the ipso-hydroxy group originated from molecular dioxygen by allowing strain Bayram to cometabolically transform 4-NP2 in an 18O2-enriched atmosphere. By means of GC-MS, we analyzed the trimethylsilyl derivative of the metabolite 2-(1-methyl-octyl)-benzene-1,4-diol, which was formed by ipso-hydroxylation and a subsequent dienone phenol rearrangement (NIH shift) (14). Comparison of the spectra of 2-(1-methyl-octyl)-benzene-1,4-diol formed in the presence and the absence of 18O2 clearly showed that the metabolite had incorporated an oxygen atom from molecular dioxygen (Fig. 4). The same result was obtained with the ipso-hydroxylated metabolites 4-hydroxy-4-(1-methyl-octyl)-cyclohexa-2,5-dienone and 4-hydroxy-4-(1-methyl-octyl)-cyclohex-2-enone (data not shown).
FIG. 4.
Origin of the ipso-hydroxy oxygen atom. EI mass spectrum of the trimethylsilyl-protected derivative of 2-(1-methyl-octyl)-benzene-1,4-diol, which was cometabolically formed by strain Bayram from 4-(1-methyl-octyl)-phenol (4-NP2) in a natural atmosphere (A) and in an atmosphere enriched with 18O2 (18O2/16O2 ratio ≈ 95.6/4.4) (B), respectively. In comparison to the control experiment (A), the m/z value of ions containing the ipso-oxygen atom is shifted by two unities (18O-M·+/16O-M·+ ≈ 95/5) in the labeling experiment (B), demonstrating that the ipso-hydroxy oxygen atom derives from molecular dioxygen.
On the other hand, the oxygen atom of the nonanol metabolite 3,6-dimethyl-heptan-3-ol, which was formed when 4-NP112 was incubated with strain Bayram, originated from water and not from molecular oxygen (Fig. 5). To rule out the possibility of a direct exchange of the hydroxy function of the alcohol with H218O, 3,6-dimethyl-heptan-3-ol was added to a control culture containing 4-NP93 as the sole source of carbon and energy. In contrast to N93OH (Fig. 2), which was formed from 4-NP93 and whose oxygen atom clearly derived from the 18O-labeled water, the added N112OH did not incorporate any 18O, even after several days of incubation. Therefore, we conclude that the degradation of 4-NP112 by strain Bayram proceeded according to pathway 3 or 4, both of which are compatible with the incorporation of H2O into the nonanol metabolite (Fig. 1). Nevertheless, about 2% of the 3,6-dimethyl-heptan-3-ol formed contained oxygen derived from molecular oxygen. This indicates that a small fraction of the alkyl cations had reacted with the ipso-hydroxy group. This conclusion was further corroborated by an experiment with technical nonylphenol and an 18O2-enriched atmosphere (18O2/16O2 ratio ≈ 95.6/4.4), which showed that certain α,α-dimethyl-substituted nonanol metabolites, which were produced in major amounts, contained a significant amount of label (up to 13% 18O).
Degradation experiments with 4-t-butoxyphenol, a structural analogue of nonyloxyphenols with an α-tertiary alkyl moiety, strongly indicated that pathway 4 was the main route of nonylphenol degradation in strain Bayram. In these experiments, the ether oxygen of the alkoxyphenol substrate was shown to be fully retained in the t-butanol metabolite derived from the side chain; in growth experiments with 18O2 and H218O, we could not observe any incorporation of the 18O label (Fig. 6). These data clearly rule out pathway 3 as a route.
FIG. 6.
Pathway proposed for the degradation of 4-t-butoxyphenol by an ipso-substitution mechanism in Sphingobium xenophagum Bayram. Growth experiments with 18O-labeled oxygen and water show that the ether oxygen in the alkoxyphenol was fully retained in the t-butanol metabolite. AH2 and A, an unidentified pair of reduced and oxidized cosubstrates, respectively.
DISCUSSION
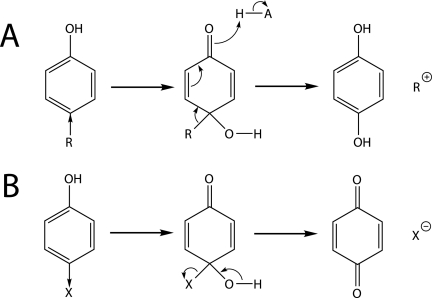
ipso-Substitution is an important mechanism by which cytochrome P450 model systems, liver microsomes, and microorganisms detach various substituents in ortho- and para-substituted phenols and anilines (15, 30). Depending on its affinity for electrons, the attached group leaves formally either as a cation or an anion (Fig. 7), and several enzymatic systems are known to detach electron-donating as well as electron-withdrawing substituents (3, 7, 10, 19, 22, 24, 30, 39, 42). However, most studies that consider cationic leaving groups refer to hydrogen substituents (3, 7, 10, 19, 22, 24), and it was only recently proposed that α-quaternary alkyl side chains are able to detach in this way (6, 14, 39). In growth and O2-monitoring experiments, S. xenophagum Bayram was able to act upon both α-quaternary 4-alkylphenols and 4-alkoxyphenols (Fig. 3). The degradation of 4-t-butoxyphenol clearly involved an ipso-substitution type of mechanism, as indicated by the retention of the ether oxygen in the t-butanol metabolite (Fig. 6) (4-nonyloxyphenols are most likely transformed analogously [Fig. 1, pathways 1 and 2]). ipso-Substitution has also been proposed for enzymatic cleavage of alkoxyphenols (3, 18, 26, 27). In contrast to oxidative O dealkylation, a well-known metabolic pathway for ether compounds involving hydroxylation of the carbon atom in α-position to the ether oxygen (9, 21), ipso-substitution is able to operate on aryl ether structures without α-hydrogens, such as 4-aryloxyphenols (29). It is tempting to suggest that the same ipso-hydroxylase is responsible for the transformation of both 4-alkyl- and 4-alkoxyphenols in strain Bayram, since both activities were strongly dependent on the induction of cells with nonylphenol (Fig. 3). The putative enzyme would have a remarkably wide substrate range, as activity was observed with α-quaternary 4-alkylphenols, 4-alkoxyphenols with α-primary and -tertiary alkyl moieties, and a 4-aryloxyphenol (Fig. 3).
FIG. 7.
ipso-Substitution of phenolic compounds that have para-substituents with electron-donating (A) or electron-withdrawing (B) properties (as indicated by the direction of the arrow). The substituent is eliminated formally either as a cation (A) or as an anion (B). Depending on whether the ring-substituent bond electrons in the cyclohexadienone derivative remain with the carbon ring or the substituent, hydroquinone (A) or p-quinone (B) is formed (7, 14, 24, 30, 39). Protonation of alkoxy side chains transforms them into good leaving groups (Fig. 6).
Although strain Bayram is able to degrade both 4-alkoxyphenols and 4-nonylphenols, the 18O-labeling experiments clearly show that 4-alkoxyphenols were not intermediates in the major metabolic pathway for cleavage of α-quaternary nonylphenols. In agreement with our results and in accordance with others (6, 30, 39, 42), we conclude that the cation released from the quinol intermediate preferentially reacted with a water molecule (Fig. 1, pathway 4) and not with the ipso-hydroxy group (pathways 1 to 3), as was previously assumed (14). The direct formation of hydroquinone seems energetically more favorable for the bacterium, because the two reducing equivalents needed for the conversion of p-quinone to hydroquinone can be saved. The breakdown of the quinol intermediate represents an SN1 reaction (unimolecular nucleophilic substitution), whereby the carbocation reacts with the solvent (solvolysis) and the dissociation energy is provided by the rearomatization of the leaving carbon ring. We did not detect any trace of nonene metabolites that might form by abstraction of a β-proton and concomitant formation of a double bond in a unimolecular elimination reaction (E1). This indicates that the combination of the cation with a water molecule might be concerted to a certain degree. However, several α,α-dimethyl-substituted nonanols incorporated a significant amount of O2-derived oxygen (up to 13%), suggesting the existence of a minor pathway in which the cation undergoes an alternative reaction and attacks the ipso-hydroxy group (Fig. 1, pathway 1 or 2). In strain TTNP3, this alternative reaction most likely leads to dead-end metabolites, as this particular strain seems unable to further degrade 4-alkoxyphenols (5, 6).
Several compounds other than nonylphenols, such as 4-ethylphenol (8) and hexachlorocyclohexane (28), have been shown to be microbially transformed to hydroquinone, which subsequently serves as a ring cleavage substrate. In a reducing environment, the hydroquinone formed is not expected to oxidize to p-quinone, a reactive compound that covalently binds to cellular macromolecules and induces oxidative stress (10). However, ipso-substitution of alkoxy moieties and of other electrophilic substituents inevitably results in the formation of this toxic intermediate (Fig. 7) (7, 10, 18, 34). To survive, strains must have developed efficient reducing systems to quickly convert p-quinone into hydroquinone.
Here we show that strain Bayram is able to degrade α-quaternary 4-nonylphenols and 4-alkoxyphenols by an ipso-substitution mechanism that involves an initial hydroxylation in the 4 position followed by the release of the side chain. In the case of an α-quaternary nonylphenol, the alkyl cation derived from the side chain combines with a molecule of water to yield the corresponding alcohol and hydroquinone (Fig. 1, pathway 4; Fig. 7A). The existence of a minor pathway in which the cation reacts with the ipso-hydroxy group has been inferred from the observed incorporation of a small amount of O2-derived oxygen into the nonanol metabolites. In the case of an alkoxyphenol, ipso-hydroxylation yields a hemiketal, which spontaneously dissociates to the corresponding alcohol and p-quinone (Fig. 1, pathway 2; Fig. 7B). Future work will show if the same hydroxylating system is responsible for the degradation of these different substrates.
Acknowledgments
This research was supported by the Swiss National Science Foundation within the framework of the National Research Programme NFP 50, Endocrine Disruptors: Relevance to Humans, Animals, and Ecosystems.
We thank U. Stalder and L. Bigler (Zürich University) for carrying out the MS analysis of the isotope-labeled oxygen gas. We also thank J. Bolotin (Eawag) for help with on-column GC-MS and A. Franchini and H.-U. Weilenmann (Eawag) for help with upscaling the culture volumes. We gratefully acknowledge P. Perisset (Eawag) for developing the O2-monitoring software.
Footnotes
Published ahead of print on 16 March 2007.
REFERENCES
- 1.Ahel, M., W. Giger, and M. Koch. 1994. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment. I. Occurrence and transformation in sewage treatment. Water Res. 28:1131-1142. [Google Scholar]
- 2.Bennie, D. T. 1999. Review of the environmental occurence of alkylphenols and alkylphenol ethoxylates. Water Qual. Res. J. Can. 34:79-122. [Google Scholar]
- 3.Boersma, M. G., J.-L. Primus, J. Koerts, C. Veeger, and I. M. C. M. Rietjens. 2000. Heme-(hydro)peroxide mediated O- and N-dealkylation. A study with microperoxidase. Eur. J. Biochem. 267:6673-6678. [DOI] [PubMed] [Google Scholar]
- 4.Brunner, P. H., S. Capri, A. Marcomini, and W. Giger. 1988. Occurrence and behaviour of linear alkylbenzenesulphonates, nonylphenol, nonylphenol mono- and nonylphenol diethoxylates in sewage and sewage sludge treatment. Water Res. 22:1465-1472. [Google Scholar]
- 5.Corvini, P. F. X., M. Elend, J. Hollender, R. Ji, A. Preiss, R. Vinken, and A. Schäffer. 2005. Metabolism of a nonylphenol isomer by Sphingomonas sp. strain TTNP3. Environ. Chem. Lett. 2:185-189. [Google Scholar]
- 6.Corvini, P. F. X., J. Hollender, R. Ji, S. Schumacher, J. Prell, G. Hommes, U. Priefer, R. Vinken, and A. Schäffer. 2006. The degradation of α-quaternary nonylphenol isomers by Sphingomonas sp. strain TTNP3 involves a type II ipso-substitution mechanism. Appl. Microbiol. Biotechnol. 70:114-122. [DOI] [PubMed] [Google Scholar]
- 7.Dai, M., J. B. Rogers, J. R. Warner, and S. D. Copley. 2003. A previously unrecognized step in pentachlorophenol degradation in Sphingobium chlorophenolicum is catalyzed by tetrachlorobenzoquinone reductase (PcpD). J. Bacteriol. 185:302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darby, J. M., D. G. Taylor, and D. J. Hopper. 1987. Hydroquinone as the ring-fission substrate in the catabolism of 4-ethylphenol and 4-hydroxyacetophenone by Pseudomonas putida JD1. J. Gen. Microbiol. 133:2137-2146. [Google Scholar]
- 9.Dardas, A., D. Gal, M. Barrelle, G. Sauret-Ignazi, R. Sterjiades, and J. Pelmont. 1985. The demethylation of guaiacol by a new bacterial cytochrome P-450. Arch. Biochem. Biophys. 236:585-592. [DOI] [PubMed] [Google Scholar]
- 10.den Besten, C., P. J. van Bladeren, E. Duizer, J. Vervoort, and I. M. C. M. Rietjens. 1993. Cytochrome P450-mediated oxidation of pentafluorophenol to tetrafluorobenzoquinone as the primary reaction product. Chem. Res. Toxicol. 6:674-680. [DOI] [PubMed] [Google Scholar]
- 11.Engesser, K. H., V. Strubel, K. Christoglou, P. Fischer, and H. G. Rast. 1989. Dioxygenolytic cleavage of aryl ether bonds: 1,10-dihydro-1,10-dihydroxyfluoren-9-one, a novel arene dihydrodiol as evidence for angular dioxygenation of dibenzofuran. FEMS Microbiol. Lett. 53:205-210. [DOI] [PubMed] [Google Scholar]
- 12.Fujii, K., N. Urano, H. Ushio, M. Satomi, and S. Kimura. 2001. Sphingomonas cloacae sp. nov., a nonylphenol-degrading bacterium isolated from wastewater of a sewage-treatment plant in Tokyo. Int. J. Syst. Evol. Microbiol. 51:603-610. [DOI] [PubMed] [Google Scholar]
- 13.Gabriel, F. L. P., W. Giger, K. Guenther, and H.-P. E. Kohler. 2005. Differential degradation of nonylphenol isomers by Sphingomonas xenophaga Bayram. Appl. Environ. Microbiol. 71:1123-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabriel, F. L. P., A. Heidlberger, D. Rentsch, W. Giger, K. Guenther, and H.-P. E. Kohler. 2005. A novel metabolic pathway for degradation of 4-nonylphenol environmental contaminants by Sphingomonas xenophaga Bayram. ipso-Hydroxylation and intramolecular rearrangement. J. Biol. Chem. 280:15526-15533. [DOI] [PubMed] [Google Scholar]
- 15.Guengerich, F. P. 2001. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 14:611-650. [DOI] [PubMed] [Google Scholar]
- 16.Guenther, K., V. Heinke, B. Thiele, E. Kleist, H. Prast, and T. Raecker. 2003. Response to comments on “Endocrine disrupting nonylphenols are ubiquitous in food.” Environ. Sci. Technol. 37:2624. [DOI] [PubMed] [Google Scholar]
- 17.Guenther, K., E. Kleist, and B. Thiele. 2006. Estrogen-active nonylphenols from an isomer-specific viewpoint: a systematic numbering system and future trends. Anal. Bioanal. Chem. 384:542-546. [DOI] [PubMed] [Google Scholar]
- 18.Hareland, W. A., R. L. Crawford, P. J. Chapman, and S. Dagley. 1975. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J. Bacteriol. 121:272-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain, M., B. Entsch, D. P. Ballou, V. Massey, and P. J. Chapman. 1980. Fluoride elimination from substrates in hydroxylation reactions catalyzed by p-hydroxybenzoate hydroxylase. J. Biol. Chem. 255:4189-4197. [PubMed] [Google Scholar]
- 20.Ieda, T., Y. Horii, G. Petrick, N. Yamashita, N. Ochiai, and K. Kannan. 2005. Analysis of nonylphenol isomers in a technical mixture and in water by comprehensive two-dimensional gas chromatography-mass spectrometry. Environ. Sci. Technol. 39:7202-7207. [DOI] [PubMed] [Google Scholar]
- 21.Karlson, U., D. F. Dwyer, S. W. Hooper, E. R. B. Moore, K. N. Timmis, and L. D. Eltis. 1993. Two independently regulated cytochromes P-450 in a Rhodococcus rhodochrous strain that degrades 2-ethoxyphenol and 4-methoxybenzoate. J. Bacteriol. 175:1467-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman, S. 1961. The enzymic conversion of 4-fluorophenylalanine to tyrosine. Biochim. Biophys. Acta 51:619-621. [DOI] [PubMed] [Google Scholar]
- 23.Kim, Y.-S., T. Katase, S. Sekine, T. Inoue, M. Makino, T. Uchiyama, Y. Fujimoto, and N. Yamashita. 2004. Variation in estrogenic activity among fractions of a commercial nonylphenol by high performance liquid chromatography. Chemosphere 54:1127-1134. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Le Garrec, G., I. Artaud, and C. Capeillère-Blandin. 2001. Purification and catalytic properties of the chlorophenol 4-monooxygenase from Burkholderia cepacia strain AC1100. Biochim. Biophys. Acta 1547:288-301. [DOI] [PubMed] [Google Scholar]
- 25.McLeese, D. W., V. Zitko, D. B. Sergeant, L. Burridge, and C. D. Metcalfe. 1981. Lethality and accumulation of alkylphenols in aquatic fauna. Chemosphere 10:723-730. [Google Scholar]
- 26.Moridani, M. Y., S. S. Cheon, S. Khan, and P. J. O'Brien. 2002. Metabolic activation of 4-hydroxyanisole by isolated rat hepatocytes. Drug Metab. Dispos. 30:1063-1069. [DOI] [PubMed] [Google Scholar]
- 27.Moridani, M. Y., M. Moore, R. A. Bartsch, Y. Yang, and S. Heibati-Sadati. 2005. Structural toxicity relationship of 4-alkoxyphenols’ cytotoxicity towards murine B16-F0 melanoma cell line. J. Pharm. Pharm. Sci. 8:348-360. [PubMed] [Google Scholar]
- 28.Nagata, Y., K. Miyauchi, and M. Takagi. 1999. Complete analysis of genes and enzymes for γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26. J. Ind. Microbiol. Biotechnol. 23:380-390. [DOI] [PubMed] [Google Scholar]
- 29.Ohe, T., T. Mashino, and M. Hirobe. 1994. Novel metabolic pathway of arylethers by cytochrome P450: cleavage of the oxygen-aromatic ring bond accompanying ipso-substitution by the oxygen atom of the active species in cytochrome P450 models and cytochrome P450. Arch. Biochem. Biophys. 310:402-409. [DOI] [PubMed] [Google Scholar]
- 30.Ohe, T., T. Mashino, and M. Hirobe. 1997. Substituent elimination from p-substituted phenols by cytochrome P450. ipso-Substitution by the oxygen atom of the active species. Drug Metab. Dispos. 25:116-122. [PubMed] [Google Scholar]
- 31.Pal, R., V. K. Bhasin, and R. Lal. 2006. Proposal to reclassify [Sphingomonas] xenophaga Stolz et al. 2000 and [Sphingomonas] taejonensis Lee et al. 2001 as Sphingobium xenophagum comb. nov. and Sphingopyxis taejonensis comb. nov., respectively. Int. J. Syst. Evol. Microbiol. 56:667-670. [DOI] [PubMed] [Google Scholar]
- 32.Servos, M. R. 1999. Review of the aquatic toxicity, estrogenic responses and bioaccumulation of alkylphenols and alkylphenol polyethoxylates. Water Qual. Res. J. Can. 34:123-177. [Google Scholar]
- 33.Sonnenschein, C., and A. M. Soto. 1998. An updated review of environmental estrogen and androgen mimics and antagonists. J. Steroid Biochem. Mol. Biol. 65:143-150. [DOI] [PubMed] [Google Scholar]
- 34.Spain, J. C., and D. T. Gibson. 1991. Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl. Environ. Microbiol. 57:812-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staples, C., E. Mihaich, J. Carbone, K. Woodburn, and G. Klecka. 2004. A weight of evidence analysis of the chronic ecotoxicity of nonylphenol ethoxylates, nonylphenol ether carboxylates, and nonylphenol. Hum. Ecol. Risk Assess. 10:999-1017. [Google Scholar]
- 36.Talmage, S. S. 1994. Environmental and human safety of major surfactants. Alcohol ethoxylates and alkylphenol ethoxylates. The Soap and Detergent Association, New York, NY.
- 37.Tanghe, T., W. Dhooge, and W. Verstraete. 1999. Isolation of a bacterial strain able to degrade branched nonylphenol. Appl. Environ. Microbiol. 65:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiele, B., K. Günther, and M. J. Schwuger. 1997. Alkylphenol ethoxylates: trace analysis and environmental behavior. Chem. Rev. 97:3247-3272. [DOI] [PubMed] [Google Scholar]
- 39.Vatsis, K. P., and M. J. Coon. 2002. ipso-Substitution by cytochrome P-450 with conversion of p-hydroxybenzene derivatives to hydroquinone: evidence for hydroperoxo-iron as the active oxygen species. Arch. Biochem. Biophys. 397:119-129. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler, T. F., J. R. Heim, M. R. LaTorre, and A. B. Janes. 1997. Mass spectral characterization of p-nonylphenol isomers using high-resolution capillary GC-MS. J. Chromatogr. Sci. 35:19-30. [Google Scholar]
- 41.White, R., S. Jobling, S. A. Hoare, J. P. Sumpter, and M. G. Parker. 1994. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology 135:175-182. [DOI] [PubMed] [Google Scholar]
- 42.Xun, L., E. Topp, and C. S. Orser. 1992. Diverse substrate range of a Flavobacterium pentachlorophenol hydroxylase and reaction stoichiometries. J. Bacteriol. 174:2898-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwank, L., T. C. Schmidt, S. B. Haderlein, and M. Berg. 2002. Simultaneous determination of fuel oxygenates and BTEX using direct aqueous injection gas chromatography mass spectrometry (DAI-GC/MS). Environ. Sci. Technol. 36:2054-2059. [DOI] [PubMed] [Google Scholar]