Abstract
The application of molecular genetics to pediatric soft tissue tumors has grown tremendously over the last decade. It has resulted in the identification of novel genes that have provided us with an increased understanding of oncogenesis. Furthermore, these findings have identified diagnostic and potentially prognostic factors for patient management. Molecular diagnostic techniques, such as reverse transcription PCR (RT-PCR) and fluorescence in situ hybridization (FISH), have become important tools for evaluating pediatric soft tissue tumors. By detecting characteristic fusion genes, these techniques have greatly increased the diagnostic accuracy of histopathological classification. One of the exciting promises of the development of these molecular techniques is their ability to detect micrometastasis and minimal residual disease. Monitoring of minimal residual disease in pediatric soft tissue tumors by quantitative RT-PCR may provide important prognostic information. Furthermore, the potential development of targeted therapy based on the understanding of the molecular pathology of a specific soft tissue tumor may complement existing treatments and improve disease outcome.
The field of molecular genetics of pediatric soft tissue tumors has expanded enormously over the last decade. This has resulted in the identification of novel genes that have contributed to the understanding of oncogenesis. It is now known that most genetic abnormalities associated with pediatric soft tissue tumors are chromosomal translocations resulting in novel fusion proteins (Table 1) . These fusion proteins often affect transcription factors resulting in a disruption of transcription regulation. This disruption may lead to activating inappropriate genes or inappropriately repressing some genes. These fusion genes, which are fairly specific to the associated tumors, can also serve as targets for the molecular diagnosis of respective tumors and the development of targeted therapy. The knowledge obtained from these studies has further translated into diagnostic, prognostic, and therapeutic applications for patient management. A comprehensive summary of the molecular and cytogenetic lesions associated with pediatric soft tissue tumors is presented in Table 1 .
Table 1.
Summary of Genetic/Molecular Lesions in Pediatric Soft Tissue Tumors
| Type of tumor | Chromosomal abnormality | Gene involved or fusion gene | Prevalence | FISH | RT-PCR* | Prognosis† |
|---|---|---|---|---|---|---|
| Rhabdomyosarcoma | ||||||
| Botryoid | NA | NA | NA | NA | NA | Good |
| Spindle cell | NA | NA | NA | NA | NA | Good |
| Embryonal | Gains of 2, 7, 8, 12, 13; losses of 1, 6, 9, 14, and 1750 | IGF2, GOK, PTCH TP53 | NA | NA | NA | Good |
| Alveolar | t(2;13)(q35;q14); | PAX3-FKHR | 75% | Ref. 67 | Ref. 13 | Poor‡ |
| t(1;13)(p36;q14) | PAX7-FKHR | 10% | ||||
| Undifferentiated | NA | NA | NA | NA | NA | Poor |
| Non-rhabdomyosarcoma-EWS family | ||||||
| EWS/PNET | t(11;22)(q24;q12) | EWS-FLI-1 | 85–95% | Ref. 108 | Ref. 109 | Good with type I fusion transcripts‡ |
| t(21;22)(q22;q12) | EWS-ERG | 5–10% | ||||
| t(7;22)(p22;q12) | EWS-ETV1 | <1% | ||||
| t(17;22)(q21;q12) | EWS-E1AF | <1% | ||||
| t(2;22)(q33;q12) | EWS-FEV | <1% | ||||
| Inversion of 22q | EWS-ZSG | <1% | ||||
| DSRCT | t(11;22)(p13;q12) | EWS-WT1 | >95% | NA | Ref. 24 | Poor |
| Clear cell sarcoma | t(12;22)(q13;q12) | EWS-ATF1 | 90% | Ref. 110 | Ref. 37 | Poor |
| Extraskeletal myxoid chondrosarcoma§ | t(9;22)(q22–23;q11–12) | EWS-TEC (CHN) | 75% | Ref. 111 | Ref. 43, 96 | Good |
| t(9;15)(q22;q21) | TCF12-TEC | NA | Ref. 43, 112 | |||
| t(9;17)(q22;q11) | TAF2N-TEC | 25% | Ref. 113 | |||
| Extraskeletal mesenchymal chondrosarcoma | der(13;21)(q10;q10) | NA | NA | Ref. 114 | NA | Poor |
| Non-rhabdomyosarcoma-others | ||||||
| Synovial sarcoma | t(X;18)(p11;q11) | SYT-SSX1 | 65% | Ref. 101 | Ref. 23 | Poor‡ |
| SYT-SSX2 | 35% | Ref. 101 | Ref. 23 | |||
| SYT-SSX4 | rare | Ref. 104 | ||||
| Congenital/infantile-fibrosarcoma | t(12;15)(p13;q25) | ETV6-NTRK3 | 80% | Ref. 115 | Ref. 39 | Good |
| Inflammatory myofibroblastic tumor | t(1;2)(q25;p23) | TPM3-ALK | NA | Ref. 10 | Ref. 10 | Good |
| t(2;19)(p23;q13) | TPM4-ALK | NA | Ref. 10 | Ref. 10 | ||
| t(2;17)(p23;q23) | CLTC-ALK | NA | Ref. 11 | Ref. 11 | ||
| t(2;11;2)(p23;p15;q31) | CARS-ALK | NA | Ref. 116 | Ref. 116 | ||
| Myxoid/round cell liposarcoma§ | t(12;16)(q13;p11) | TLS-CHOP | >95% | Ref. 109 | Ref. 117 | Good |
| t(12;22)(q13;q12) | EWS-CHOP | rare | Ref. 97 | |||
| Tenosynovial giant cell tumor | t(1;2)(p11;q35–37) | NA | 40% | Ref. 118 | NA | Good |
| t(1;5)(p11;q22–31) | 10% | |||||
| t(1;11)(p11;q11–12) | 10% | |||||
| t(1;8)(p11;q21–22) | 10% | |||||
| Lipoblastoma | Rearrangement of 8q12, polysomy 8 | PLAG1 | 70% 20% | Ref. 119, 126 | NA | Good |
| Malignant peripheral nerve sheath tumor | Complex changes | NA | NA | NA | NA | Poor |
| DFSP | Ring chromosome with sequences from chromosomes 17 and 22, t(17;22)(q22;q13) | COL1A1-PDGFB | >99% | Ref. 121, 122 | Ref. 38, 123 | Good |
| Desmoid tumor | +8, +20, | NA | NA | Ref. 24 | NA | Good |
| Deletion (5)(q21–22) | NA | NA | NA | NA | ||
| Alveolar soft part sarcoma | t(X;17)(p11;q25) | ASPL-TFE3 | > 90% | NA | Ref. 125 | |
| Giant cell fibroblastoma (juvenile form of DFSP) | t(17;22)(q22;q13) | COL1A1-PDGFB | NA | Refs. 122, 126 | Refs. 38, 123, 126 | |
DFSP, Dermatofibrosarcoma protuberans; NA, not applicable/not known.
The number shown is the corresponding number of references containing protocol for FISH or RT-PCR.
Prognosis based on morphologic classification.
Fusion transcripts correlate with prognosis of patients, see text for detail.
This review is divided into three sections: the application of molecular techniques in clinical management, technical considerations for the commonly used molecular diagnostic techniques, and a brief review of the molecular genetics/pathogenesis of the most common and well-defined pediatric soft tissue tumors.
Application of Molecular Genetics in Clinical Management
Diagnostic Applications
For the purpose of clinical management, pediatric soft tissue tumors are broadly divided into: rhabdomyosarcomas and non-rhabdomyosarcomas (Ewing/peripheral primitive neuroectodermal tumor (PNET) and other sarcomas). Rhabdomyosarcomas are treated primarily with chemotherapy. 1, 2 The role of surgery is limited to initial biopsy, wide local excision (whenever clear margins are possible), and resection of residual disease. Radiotherapy in the form of external beam or brachytherapy is restricted to persistent or recurrent disease. The primary therapy for non-rhabdomyosarcomas is surgical resection, but adjuvant radiotherapy and chemotherapy are being used with increasing success. 1, 3 Another major difference between these two categories is involvement of lymphatics. Rhabdomyosarcomas often involve the regional lymph nodes, indicating the importance of lymph node evaluation for staging. Non-rhabdomyosarcomas involve lymph nodes less commonly and the spread of these tumors is predominantly hematogenous.
As outlined above, the accurate diagnosis of pediatric soft tissue tumors is critical for clinical management. Accurate diagnosis requires integration of clinical findings (age, sites of involvement, pattern of disease spread, and radiographical characteristics), morphological evaluation, and ancillary tests including immunohistochemistry, cytogenetics, and molecular genetics.
Molecular diagnostic techniques, particularly RT-PCR and FISH, have become important tools to detect the characteristic fusion genes associated with pediatric soft tissue tumors (Table 1) since these molecular techniques require only a minimal amount of tissue. Recently, diagnostic procedure has been changed from open incisional biopsy to methods, such as core biopsy and fine-needle aspirate biopsy (FNAB), requiring smaller amounts of tissue without compromising the accuracy of diagnosis. Such methods are easily accepted by patients, may be performed in ambulatory clinics, and are less likely to have significant complications. The success of these methods (core biopsy and FNAB) relies heavily on the prudent use of the small amount of tissue obtained.
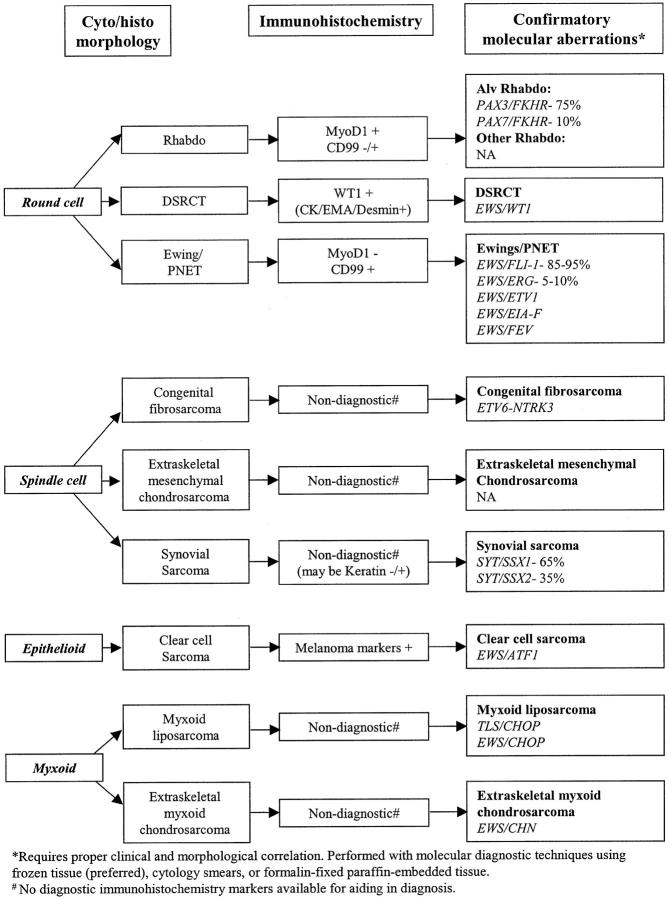
The contributions of molecular genetics have significantly improved the accuracy of diagnosis of pediatric soft tissue tumors. 4, 5, 6, 7 A practical diagnostic approach of integrating morphology, immunohistochemistry, and molecular genetics using small amounts of tissue is illustrated in Figure 1 with representative examples. The specimens are evaluated for adequacy during FNAB and/or core biopsy by immediate morphological interpretation of the cytology smear or frozen section. The initial differential diagnosis based on morphology evaluation is further refined using immunohistochemistry (Table 2) . In the majority of cases, the above evaluation may be sufficient for diagnosis. In difficult cases such as monophasic synovial sarcoma with spindle cell pattern, molecular genetics can be used. Furthermore, whenever there is doubt due to either the atypical morphology or the unexpected immunohistochemistry findings, molecular genetic study may be performed for further evaluation. The exact nature of molecular targets to be tested will depend on the initial differential diagnosis based on morphology and immunohistochemistry as illustrated in Figure 1 .
Figure 1.
Algorithm highlighting diagnostic contribution by molecular genetic study. Several examples are shown for illustrating purpose. For details please see text, Tables 1 and 2 .
Table 2.
Differential Diagnosis of Pediatric Soft Tissue Tumors Based on Cyto/Histomorphology and Immunohistochemistry
| Cyto/histomorphology | Soft tissue tumor | Diagnostic immunoprofile |
|---|---|---|
| Round cell | Rhabdomyosarcoma | MyoD1+ |
| PNET | CD 99 + | |
| DSRCT | WT1+, keratin+, EMA+, Desmin+ | |
| Spindle cell | Fibromatosis | Non-diagnostic |
| Spindle cell lipoma | Non-diagnostic | |
| Schwannoma/neurofibroma | S-100 protein+ | |
| Kaposi’s sarcoma* | CD34+, CD31+ | |
| Dermatofibrosarcoma protuberans | CD 34+ | |
| Fibrosarcoma* | Non-diagnostic | |
| Leiomyosarcoma* | SMA +, Desmin + | |
| Malignant peripheral nerve sheath tumor | S-100 protein + (weak)/- | |
| Malignant fibrous histiocytoma (some)* | Non-diagnostic | |
| Synovial sarcoma | Usually non-diagnostic (Keratin −/+, EMA −/+) | |
| Angiosarcoma (some)* | CD 31 +, CD 34 +, Factor VIII + | |
| Epithelioid (polygonal) cell | Epithelioid hemangioma Granular cell tumor | CD 31 +, CD 34 +, S-100 protein+ |
| Clear cell sarcoma | Melanoma markers + | |
| Epithelioid sarcoma | CD 34 +, CK + | |
| Alveolar soft part sarcoma | Non-diagnostic | |
| Angiosarcoma (some)* | CD 31 +, CD 34 +, Factor VIII + | |
| Myxoid | Nodular fasciitis | Non-diagnostic |
| Myxoma | Non-diagnostic | |
| Myxoid liposarcoma* | Non-diagnostic | |
| Myxoid chondrosarcoma* | Non-diagnostic | |
| Myxoid malignant fibrous histiocytoma | Non-diagnostic | |
| Adipocytes | Lipoma | Non-diagnostic |
| Lipoblastoma | Non-diagnostic | |
| Well differentiated liposarcoma* | Non-diagnostic |
Although different opinions exist regarding how to weigh the impact of molecular genetics testing results on diagnosing pediatric soft tissue tumors, most authorities concur that conventional morphological assessment, supplemented by ancillary techniques including molecular genetics, remains the standard diagnostic approach. 4, 5, 6, 7 This is because the specificity of fusion genes, although reasonably high, is not absolute. A good example is that TPM3-ALK and CLTC-ALK gene fusions have been recently identified in both inflammatory myofibroblastic tumors and anaplastic large cell lymphomas. 8, 9, 10, 11 An appropriate guideline for handling discrepant histopathological findings and molecular genetics results has been recently suggested by Ladanyi et al 6 Briefly, if a gene fusion is absent in cases of tumor type typically having the gene fusion, the quality of molecular diagnostic methods should be further evaluated to avoid false-negative results. In contrast, if a given gene fusion is present by one molecular diagnostic test in cases of tumor type typically not having the gene fusion, other molecular diagnostic tests should be performed to exclude false-positive interpretation.
Prognostic Applications
Prognostic Significance of Fusion Proteins
Studies have suggested that the different fusion proteins in each specific soft tissue tumor may have prognostic significance. Anderson et al 12 recently reported the translocation, t(2;13)/PAX3-FKHR, to be an adverse prognostic factor for alveolar rhabdomyosarcoma. In contrast, t(1;13)/PAX7-FKHR was associated with a favorable prognosis and was more frequently observed in younger patients with relatively localized disease. 12 Sorensen et al 13 reported that, among the patients with metastatic alveolar rhabdomyosarcoma, bone marrow involvement was significantly higher in PAX3-FKHR-positive patients. Furthermore, in patients presenting with metastatic disease, there was a striking difference in outcome between PAX7-FKHR and PAX3-FKHR patient groups (estimated 4-year overall survival rate of 75% for PAX7-FKHR versus 8% for PAX3-FKHR). 13
Similarly, recent studies have suggested that the most common type of EWS-FLI1 fusion transcript, type 1 (EWS exon 7 fused to FLI-1 exon 6), is associated with a favorable prognosis and appears to encode a functionally weaker transactivator, compared to other types of fusion transcripts. 14, 15 Among patients with localized disease, patients with a type 1 fusion product had a longer relapse-free survival than those with other variants of fusion transcripts. 14 Finally, in localized synovial sarcoma, patients with the SYT-SSX2 fusion variant had significantly longer metastasis-free survival than those with SYT-SSX1 variant. 16 Ladanyi et al 17 recently reported that the patients with the SYT-SSX2 fusion variant had better overall survival than those with SYT-SSX1 variant.
Even more promising is the potential ability of gene expression profiles by cDNA microarray to detect different prognostic groups. In a pilot study, using cDNA microarray and cluster analysis, the gene expression profiles of different types of pediatric soft tissue tumors correctly discriminated patients who survived from those who did not, with P values less than 0.0001. 18 Notably, none of the existing prognostic factors, including histological type and response to therapy, were as reliable for predicting the survival in these patients. 18
Prognostic Significance of Detection and Quantitation of Minimal Disease
The sensitivity and specificity of the RT-PCR protocols have provided evidence for the existence of minimal disease which cannot be detected by physical/morphological/radiographical examination. Cumulative data has demonstrated that most patients still harbor a significant tumor burden after resection. When patients with Ewing’s sarcoma or PNET were studied using RT-PCR, while about 50% of patients with metastatic or relapsed disease had blood or marrow samples positive for the EWS-FLI-1 transcripts; approximately 25% of patients without clinically metastatic disease did so as well. 19 Similarly, using RT-PCR for detecting the PAX3-FKHR and PAX7-FKHR transcripts, Kelly et al 20 reported that some marrow samples, from patients with alveolar rhabdomyosarcoma, interpreted as negative by light microscopy were positive by RT-PCR. Recently, detection of fetal acetylcholine receptor by RT-PCR has shown to be a reliable marker for detecting minimal disease for both alveolar and embryonal rhabdomyosarcomas. 21 Other RT-PCR methods to detect minimal disease of synovial sarcoma and desmoplastic small round cell tumors have also been described. 22, 23, 24 However, the clinical significance of minimal disease remains to be evaluated. 22, 24
It is anticipated that, similar to hematopoietic malignancies, monitoring of minimal residual disease by quantitative RT-PCR will, in the future, provide important prognostic information for the management of pediatric soft tissue tumors. 25, 26 In patients with chronic myelogenous leukemia, rising or persistently high levels of BCR-ABL (BCR-ABL:ABL ratio of >0.02% or >100 BCR-ABL transcripts/μg RNA), determined by quantitative RT-PCR, in two sequential specimens more than 4 months following stem cell transplantation, are predictive of overt clinical relapse. 26 Similarly, using real-time quantitative PCR to quantify the amount of t(14;18)(q32;q21) cells, we have recently observed that measurable tumor load ≥ 0.01% after stem cell transplantation may predict subsequent relapse of follicular lymphoma (Chang C, Bredeson C, Juckett M, Logan B, Keever-Taylor CA, manuscript submitted). Furthermore, studies have shown that treating CML patients before overt clinical relapse of disease can improve survival of CML patients after stem cell transplantation. 27 Quantitation of minimal residual disease may lead to new therapeutic avenues to improve patient outcome in pediatric soft tissue tumors. Furthermore, quantitation of minimal residual disease has become clinically practical due to the recent emergence of devices for performing real-time RT-PCR.
Therapeutic Applications
Targeted therapy potentially can be developed based on the understanding of the molecular pathology of pediatric soft tissue tumors. 18, 28, 29, 30, 31, 32, 33, 34 For example, when the gene expression profiles of metastatic tumors were compared to that of non-metastatic and favorable tumors by cDNA microarray, several candidate genes that represent potential therapeutic targets were readily identified. 18 These targets are analogous to the HER2/neu receptor in breast cancer and c-Kit in gastrointestinal stromal tumors. Allander et al 35 reported the identification of a new potential therapeutic target, ERBB2 (HER2/neu), for a subset of synovial sarcoma cases using cDNA microarray analysis.
Fusion proteins generated by chromosomal translocations are true tumor-specific antigens and are promising targets for immunotherapy. 31 In a recent study, the induction of synovial sarcoma-specific cytotoxic T-lymphocytes from normal donor lymphocytes using in vitro stimulation with fusion peptide (derived from SYT-SSX fusion protein)-pulsed dendritic cells has been demonstrated. These cytotoxic T-lymphocytes have the ability to lyse human synovial sarcoma tumor cells expressing the fusion protein. 36 These findings suggest that a peptide derived from the fusion protein may work as a neoantigen and induce a tumor-specific immune response.
Other molecular targets currently under investigation include: antisense oligonucleotides to the fusion transcripts, signal transduction inhibitors, angiogenesis inhibitors, matrix metalloproteinase inhibitors, inhibitors of fusion protein, and differentiation inducers. 29, 30, 32 These targeted approaches along with traditional therapeutic modalities will be valuable additions to the armamentarium of current methods for tumor management.
Technical Considerations of the Commonly Used Molecular Diagnostic Techniques
Although most of the characteristic chromosomal translocations in the soft tissue tumors can be detected by conventional cytogenetics, molecular diagnostic techniques have become an important complementary tool for detecting these fusion genes. Cytogenetics studies require fresh samples that may not always be available. Furthermore, cytogenetic studies are limited by growth failure of tumor cells, masking of tumor cells by overgrowth of karyotypically normal stromal cells, and long turnaround time (2 to 7 days).
In clinical laboratories, the most common molecular diagnostic techniques used to detect these fusion genes are RT-PCR (qualitative and/or quantitative) and FISH, although other methods can be applied as well (see below). Molecular studies require a minimum of 100 mg of representative viable tumor tissue frozen (at − 80°C in OCT embedding compound, Sakura Tissue Tec, Torrance, CA, or snap-frozen in liquid nitrogen) and stored in a −80°C freezer. Technologies such as comparative genomic hybridization (CGH), spectral karyotyping (SKY), multifluorophore fluorescence in situ hybridization (M-FISH), and cDNA microarray remain predominantly research tools to investigate the oncogenesis of these tumors.
Southern Blot
Southern blot is the oldest nucleic acid-based method and is used extensively in research settings. It is a simple but powerful basic tool for molecular studies. Using a probe targeting a specific oncogene, this method is particularly useful in detecting a translocation when the partners of a translocation of a specific gene are multiple or unknown. Furthermore, this method can quantitatively determine the number of copies of a gene when compared with an appropriate control. However, this method suffers from the disadvantages of a turnaround time of 4 to 5 days. It further requires a relatively large quantity (micrograms) of fresh DNA. Thus, this method cannot be applied when only a small amount of tissue sample is available (such as in needle core biopsies) or when freshly frozen samples are not stored. Ultimately, these factors discourage its application for diagnostic purposes.
RT-PCR-Based Methods
Most characteristic translocations reported in pediatric soft tissue tumors have genomic breakpoints located more or less randomly within large introns. This precludes using DNA to detect these translocations by PCR, although DNA is easier to handle and more readily retrieved from paraffin-embedded tissue than RNA. Nevertheless, the breakpoints of these translocations are clustered enough so that primers can be designed to detect most of the translocations using RT-PCR (Table 1) .
The RT-PCR-based methods have become the most practical method for detecting fusion transcripts. The advantage of RT-PCR-based methods is that only a small amount of tissue is required. However, preservation of good quality RNA is essential. Thus, appropriate handling of tissue samples is critical. For RT-PCR, tissue can be snap-frozen in liquid nitrogen or be immediately immersed in OCT embedding compound and then snap-frozen with dry ice or liquid nitrogen. Both methods preserve adequate quality RNA for RT-PCR (conventional and real-time quantitative RT-PCR); however, embedding tissue in OCT compound is more readily combined with microdissection technique. The recently available RNA, RNAlater (Ambion RNA Diagnostics, Austin, TX), provides an alternative way to preserve RNA when immediate freezing is not possible. The quality of RNA preserved with RNAlater is comparable to that preserved with the above two methods (personal experience). Although RNA extracted from paraffin-embedded tissue has been used for RT-PCR to detect fusion transcripts, 37, 38, 39, 40, 41, 42, 43 the diagnostic yield with this approach may be inferior. 7 A recent multi-center study revealed significantly decreased diagnostic yield of IgH gene rearrangement when paraffin-embedded tissue was used. 44 Fresh-frozen tissue, preferably containing a minimum of 100 mg (approximately 5 × 5 × 5 mm) of viable tumor, remains the most favorable testing material.
The major disadvantage of RT-PCR is the need for scrupulous laboratory practices to avoid contamination of locations and equipment with PCR products. In our laboratory, we have successfully used uracil-N-glycosylase (UNG) to reduce contamination problems without compromising the efficiency of real-time PCR. Furthermore, vigorous negative controls including “no RNA (water)” and “no reverse transcriptase” controls should be included in the RT-PCR assays. The former is to evaluate reagent contamination and the latter is to address the possibility of contamination of the RNA samples themselves.
In addition to detection, quantitative RT-PCR can also be used for the quantitation of fusion transcripts. The conventional quantitative RT-PCR techniques by either competitive or limiting dilution methods are too labor intensive for clinical practice. The recently developed real-time quantitative RT-PCR technology using fluorescence resonance energy transfer (FRET) is easy to perform with quick turnaround time. 45, 46, 47, 48, 49 Assay time, including RNA extraction, is about 6 hours, compared with 2 days for standard RT-PCR methods. The assay time can be further shortened to 4 hours if an instrument capable of rapid cycling, such as LightCycler (Roche Diagnostics, Indianapolis, IN), is used. 45 It is likely that this technology will become the mainstream method for detecting the fusion transcripts and quantitating their levels. 46, 47, 48, 49
Recently, Peter et al 49 reported the development of a multiplex real-time RT-PCR which enabled the detection with identical PCR conditions of the different fusions specifically observed in Ewing tumors, alveolar rhabdomyosarcoma, synovial sarcoma, small round cell desmoplastic tumors, extraskeletal myxoid chondrosarcoma, malignant melanoma of soft parts, and congenital fibrosarcoma. The multiplex reaction can reduce cost and turnaround time significantly. Furthermore, this type of technology constitutes an important step toward the complete automation of detecting cancer-specific gene fusions.
Fluorescence in Situ Hybridization
Fluorescence in situ hybridization (FISH) offers several advantages over Southern blot and PCR-based methods. FISH has the ability to examine individual cells. Thus, it can potentially distinguish tumor cells from normal cells and detect heterogeneity within tumor cells. However, this may be a difficult task at times. Interphase FISH can be applied to cytology smears and histological sections from formalin-fixed, paraffin-embedded tissue. 6 However, FISH is more time consuming and labor intensive than RT-PCR. Surprisingly, despite the importance of detecting fusion genes in the diagnosis of pediatric soft tissue tumor, there are no commercially available FISH probes for the vast majority of these genes. The applications of FISH in various soft tissue tumors as reported in the literature are summarized in Table 1 .
Comparative Genomic Hybridization
Comparative genomic hybridization (CGH) is particularly useful for the detection of yet unidentified genomic regions that are amplified or deleted in a particular tumor. 50, 51, 52, 53, 54, 55, 56, 57 For example, studies using this technology have generated significant clues in the oncogenesis of rhabdomyosarcoma, 50, 51 clear cell sarcoma, 58 synovial sarcoma, 55 and Ewing tumors. 53 The major limitation of CGH is that it cannot detect low copy number amplifications, small deletions, and balanced rearrangements such as inversions or translocations. This method requires imaging hardware and software that is present in most commercially available systems.
Spectral Karyotyping (SKY)
Using spectral karyotyping (SKY), a universal chromosome painting technique, all of the chromosomes can be labeled at once, providing a comprehensive FISH screening test for detecting cytogenetic changes. 59 This technology recognizes extremely complex or subtle gene rearrangements. This technology is based on metaphase analysis and can only be performed on viable tissue. A related technology, multi-fluorophore fluorescence in situ hybridization (M-FISH), has also been developed. 60, 61 Both methods are limited by the inability to detect intrachromosomal anomalies, abnormalities involving the p-arms of acrocentrics, and areas rich in highly repetitive DNA. 61
cDNA Microarray
Preliminary works have shown promise for classifying soft-tissue tumors based on gene expression profiles using cDNA microarray, which have the capability of simultaneously examining the expression of more than 12,000 genes. 18, 62, 63, 64, 65 For example, Khan et al 65 have recently shown that alveolar rhabdomyosarcoma cells show a characteristic pattern of gene expression, which allows this group of tumor to be identified by cDNA microarray analysis. Triche 18 reported preliminary results indicating that simple hierarchical clustering analysis of gene expression profiles by cDNA microarray categorized all but two of 23 “small round cell tumors” of childhood. With more sensitive clustering methods for the analysis of gene expression profiles, subcategories of tumors (such as embryonal versus alveolar rhabdomyosarcoma) and even subsets of alveolar RMS (such as with a PAX3/FKHR, PAX7/FKHR, or without any translocation) could be readily distinguished. 18 Additionally, this technology has been explored for predicting prognosis of pediatric soft tissue tumors and for developing targeted therapy (see above). The power of this technology can be further enhanced with laser microdissection technology to select a pure tumor population. Further studies are required to determine the clinical role of the cDNA microarray technology.
Brief Review of Molecular Genetics/Pathogenesis of the Most Common and Well-Defined Pediatric Soft Tissue Tumors
Rhabdomyosarcoma
Rhabdomyosarcoma is the most common type of pediatric soft tissue sarcoma, comprising about 50% of cases. Its subtypes vary according to histological appearance, clinical presentation, and outcome. The major morphological subtypes are alveolar (20% of cases) and embryonal (65% of cases). Survival may vary from greater than 95% (embryonal subtype) to less than 25% (alveolar subtype). 66 The contributions of molecular genetics have significantly improved the accuracy of diagnosis within subtypes and enhanced the understanding of oncogenesis.
Alveolar Rhabdomyosarcoma
A characteristic translocation, t(2;13)(q35;q14), is seen in about 75% of cases. 67, 68, 69, 70 With this translocation, the PAX3 gene on chromosome 2 is fused with the FKHR (also known as FOX10A or ALV) gene on chromosome 13, resulting in a chimeric transcription factor derived from the 5′ end of the PAX3 gene and the 3′ end of FKHR gene (exons 2 and 3). 70 Both portions encode DNA-binding domains. A less frequently associated translocation, t(1;13)(p36;q14), is observed in 10% of cases. With this translocation the PAX7 gene on chromosome 1 is involved. Recently, Barr et al 71 reported several genetically distinct subsets of alveolar rhabdomyosarcoma (ARMS) cases without characteristic PAX3-FKHR or PAX7-FKHR transcripts. These subsets include cases with low expression or atypical presentation of standard fusions, variant fusions with other genes, and true fusion-negative cases.
The PAX3 and PAX7 genes belong to a family of PAX genes that encode transcription factors important in normal embryonic development. 72 Both of these genes encode DNA-binding domains, referred to as “paired box” and “homeobox” domains. PAX3 and PAX7 are highly homologous, suggesting similar functions. Both genes are expressed in developing myotomes, which may explain the involvement of these genes in rhabdomyosarcoma. The FKHR gene is a member of a different family of genes that also encode transcription factors involved in the developing embryo.
The fusion proteins encoded by the PAX3/FKHR and PAX7/FKHR transcripts are similar. The DNA-binding domains of PAX3 and PAX7 are retained in the fusion proteins. 73 The FKHR portion of the fusion protein, although it has lost its DNA-binding domain, contributes an activation domain that is lost from the native PAX3 or PAX7 proteins as a result of the translocation. Thus, the activation domain of the FKHR protein may lead to aberrant levels of expression of the target genes of PAX3 or PAX7. This aberrant gene expression may affect the control of growth, apoptosis, differentiation, and motility of myogenic precursor cells. 73 In a recent study, the expression levels of Itm2A, Fath, FLT1, TGFA, BVES, and EN2 genes correlated with the levels of PAX3-FKHR expression in rhabdomyosarcoma cell lines. These genes may be part of the PAX3-FKHR regulatory pathway during tumor formation. 74
Embryonal Rhabdomyosarcoma
“Tumor-specific” genetic changes have not been reported in embryonal rhabdomyosarcoma. However, consistent/recurrent cytogenetics changes (+2, +7, +8, +11, +12, etc) have been observed. This pattern of changes may be helpful in classifying the lesion, although it is not definitive or tumor specific. Recently, using comparative genomic hybridization (CGH), Bridge et al 50 reported gains of chromosomes or chromosomal regions [2 (50% of cases), 7 (42%), 8 (67%), 11 (42%), 12 (58%), 20 (33%) and 13q21] and losses of 1p35–36.3 (42%), 6 (33%), 9q22 (33%), 14q21–32 (25%), and 17 (25%) as the most common genetic abnormalities in embryonal rhabdomyosarcoma. Loss of heterozygosity at chromosome 11p15 leading to overexpression of IGF2 (insulin growth factor 2) through loss of imprinting or paternal disomy has been reported in some cases. 75 Chromosome fragment transfer studies have further suggested the presence of other unknown tumor suppressor gene(s) in chromosome 11p15. 69 Most recently, Bridge et al 51 reported that genomic amplification was detected, by CGH, in 23% of embryonal rhabdomyosarcoma cases with anaplastic features. One amplicon, corresponding to the locus of the insulin-like growth factor type I receptor (IGF1R) gene located at 15q25–26, may play a role in the development or progression of this subset of rhabdomyosarcomas. Finally, p53 mutation may have a role in the development of rhabdomyosarcoma. 50 Rhabdomyosarcoma is the most common sarcoma observed in Li-Fraumeni syndrome. The majority of patients with this syndrome demonstrate a germ-line mutation in p53.
Ewing’s Sarcoma and Peripheral Primitive Neuroectodermal Tumor
The shared genetic anomalies have confirmed a common histopathogenesis between these histopathologically similar tumors. Both tumors are associated with a reciprocal translocation involving chromosome 22 at q12. 76, 77, 78 In about 90% of cases, the other involved chromosome is 11 at q24. 79 This translocation fuses the FLI-1 gene (on chromosome 11) with the EWS gene (on chromosome 22), resulting in a chimeric protein derived from the 5′ end of EWS and 3′ end of FLI-1. The breakpoints are variable with at least 10 different transcripts of different sizes, ranging from 300 to 700 bps, detectable by RT-PCR. 80 The most frequent fusion transcripts join exon 7 of EWS to either exon 6 or exon 5 of FLI-1. They are designated as type 1 and type 2 fusion transcripts, respectively. 81 The type 1 fusion transcript is associated with a favorable prognosis and appears to encode a functionally weaker transactivator as compared to other fusion types. 15, 81
The normal function of the EWS gene remains to be defined, although it is ubiquitously expressed. Ewing’s sarcoma (EWS) is believed to function as an adaptor between the RNA polymerase II transcription complex and RNA splicing factors. 77, 82 The FLI-1 gene is a member of the ETS family of proto-oncogenes and contains a DNA-binding domain and functions as a transcription activator. The fusion protein retains the polymerase II-interacting domain but loses the C-terminal domain of EWS necessary for normal interaction with splicing factors. Studies have shown that the fusion protein inhibits YB-1 mediated splicing of pre-mRNA and that the ability to inhibit pre-mRNA splicing coincides with the transforming ability of this fusion protein in vitro. 77, 83, 84 Furthermore, the fusion protein acts as a transcriptional activator or repressor of a set of target genes identified by microarray analysis. 85 These findings suggest that the fusion protein plays a critical role in oncogenesis. Finally, studies have shown that the p53 pathway and the basic fibroblast growth factors (bFGF) pathway may play an important role in modulating fusion protein oncogenicity. 77, 86 P53 alteration appears to define a small clinical subset of patients with EWS/PNET with a markedly poor outcome. 87
To date, at least four additional but less common translocations have been reported in Ewing’s sarcoma and PNET (Table 1) . All of them maintain a common theme of fusing a portion of the EWS gene to another gene similar to the FLI-1 gene. 76 All these genes belong to the ETS family of transcription factors. Among these translocations, t(21;22)(q22;q12) is the most common and is seen in up to 5% of cases. Some variants with more complex translocations involving more than two chromosomes have been reported, but the EWS gene seems to be a consistent feature. 88 Recently, a novel fusion gene, EWS/ZSG, caused by inversion of 22q has been reported. 89 However, the definite interpretation of this tumor appears to be controversial since this tumor has overlapping histological features of desmoplastic small round-cell tumor (DSCRT) and PNET.
Desmoplastic Small Round-Cell Tumor
DSRCT, originally described as intra-abdominal DSRCT, is a polyphenotypic tumor with a tendency for widespread peritoneal involvement. DSRCT occurs predominantly in young males and has a distinctive histological appearance. Recently, the spectrum of DSCRT has been expanded to include other histological variants and other sites including the ovary, pleura, and even the posterior cranial fossa. 6, 90
In DSRCT, the EWS gene on chromosome 22 is fused with the WT1 gene (Wilms tumor suppressor gene) on chromosome 11. 91 The resulting chimeric protein is derived from the 5′ end of the EWS gene and the 3′ end of the WT1 gene. As in Ewing’s sarcoma, the fusion gene in DSRCT includes up to exon 7 and rarely exon 8, 9, or 10 of EWS. 92 The fusion includes exons 8–10 of WT1 that encode most (but not all) of the portion of the DNA-binding domain. The WT1 gene is normally expressed in kidney during the time of its transition from a mesenchymal phenotype to an epithelial phenotype. 93, 94 This gene is also expressed in mesothelium, which may lead to involvement of mesothelium in DSRCT and the polyphenotypic nature of DSRCT. 93, 94 In DSRCT, the retained portion of WT1 behaves as a transcriptional activator, acting on some of the target genes of the normal WT1 protein. 93 For example, it has been shown that expression of EWS-WT1 induces the expression of endogenous platelet- derived growth factor-A (PDGFA), whose promoter contains many potential WT1-binding sites. 95 The induction of PDGFA, a potent fibroblast growth factor, may contribute to the characteristic reactive fibrosis associated with this unique tumor. 95
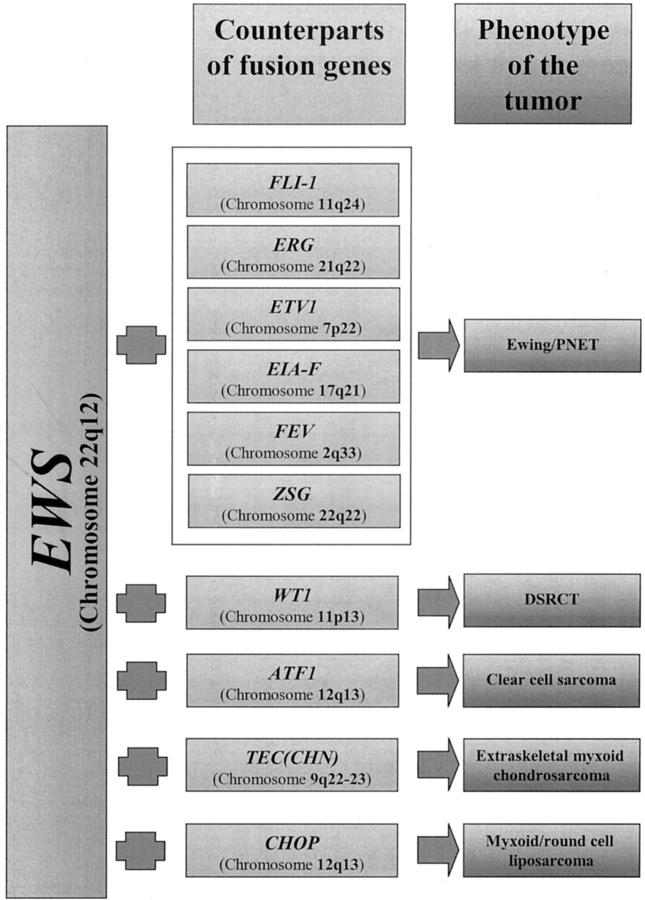
In addition to Ewing’s sarcoma/PNET and DSRCT, the fusion of EWS gene is observed in clear-cell sarcoma, 37 extraskeletal myxoid chondrosarcoma, 43, 96 and myxoid/round cell liposarcoma, 97 though the counterpart genes in all these tumors are different (Figure 2) . This suggests that the fusion partner may determine the tumor phenotype. The fusion partner’s DNA-binding properties may determine which target genes are activated, thus giving rise to a specific tumor phenotype. 98, 99
Figure 2.
The phenotype of the tumor may vary according to the fusion partners of EWS gene.
Synovial Sarcoma
A characteristic translocation t(X;18)(p11;q11), has been identified in up to 95% of cases of synovial sarcoma. 66, 100 In the vast majority of cases, this translocation leads to a fusion transcript of the 5′ portion of the SYT gene on chromosome 18 with the 3′ portion of one of two genes, SSX1 or SXX2, on chromosome X. 23, 40, 101 Both monophasic (purely mesenchymal morphology) and biphasic (with both epithelial and mesenchymal features) variants of synovial sarcoma are associated with this translocation. Most synovial sarcomas with SYT/SSX1 show biphasic morphology; in contrast, most synovial sarcomas with SYT/SSX2 show monophasic morphology. 40 Recently, fusion transcript variants of a novel fusion gene SYT/SSX4 have been reported. 102, 103, 104
The function of these fusion proteins remains to be defined. In the SYT/SSX fusion transcript, the potential transcriptional activator domain of SYT gene replaces the potential transcriptional repressor domain of the SSX genes. Hypothetically, this could lead to aberrant expression of SSX target genes. In one recent study, SYT-SSX was shown to stabilize cyclin D1 and was critical for cyclin D1 expression in synovial sarcoma cells. 105 SYT/SSX-dependent expression of cyclin D1 may be a significant event in the development and progression of synovial sarcoma. 105 Additionally, Nagai 106 recently reported that the N-terminal 181 amino acids of SYT-SSX1 were essential for the transformation of cultured fibroblasts and were shown to mediate binding to the chromatin remodeling factor hBMR/hSNFα. For further comprehensive review of molecular genetics of synovial sarcoma, the reader is referred to a recent article by Sandberg and Bridge. 107
Conclusion
Molecular diagnostic techniques to detect characteristic fusion genes have proved to be of significant importance as a tool for evaluating pediatric soft tissue tumors. The detection of these fusion genes has strengthened existing concepts of oncogenesis and greatly enhanced diagnostic accuracy. One of the exciting promises of the molecular techniques is the ability to detect fusion genes in the setting of micrometastasis and minimal residual disease. In the future, the challenge for the medical community is to establish the prognostic significance of micrometastasis and minimal residual disease. To surmount this challenge, it is important to standardize the RT-PCR methods used to detect and quantitate the characteristic fusion transcripts within the molecular pathology community. Furthermore, the potential development of targeted therapy based on the understanding of the molecular pathology of specific soft tissue tumor may further complement the existing treatments to improve the disease outcome.
Acknowledgments
We thank Dr. Karl V. Voelkerding, Advisor for Molecular Development, Associated Regional University Pathologists (ARUP) Laboratories, Salt Lake City, Utah, and Dr. Peter van Tuinen, Director of Cytogenetics Laboratory, Department of Pathology, Medical College of Wisconsin, Milwaukee, Wisconsin, for their critical review and thoughtful inputs of this manuscript. We also thank Drs. Louis Novoa-Takara and Anthony Cafaro for their editorial comments.
Address reprint requests to Chung-Che Chang, M.D., Ph.D., Department of Pathology, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030. E-mail:jeffchang@pol.net.
Footnotes
Supported by College of American Pathologist Scholar Program (to C.C.) and Cancer Center Development Grant, Medical College of Wisconsin (to C.C. and V.S.).
References
- 1.Raney RB: Soft-tissue sarcoma in childhood and adolescence. Curr Oncol Rep 2002, 4:291-298 [DOI] [PubMed] [Google Scholar]
- 2.Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, Breneman J, Qualman SJ, Wiener E, Wharam M, Lobe T, Webber B, Maurer HM, Donaldson SS: Intergroup rhabdomyosarcoma study-IV: results for patients with non-metastatic disease. J Clin Oncol 2001, 19:3091-3102 [DOI] [PubMed] [Google Scholar]
- 3.Burdach S, Jurgens H: High-dose chemoradiotherapy (HDC) in the Ewing family of tumors (EFT). Crit Rev Oncol Hematol 2002, 41:169-189 [DOI] [PubMed] [Google Scholar]
- 4.Pfeifer JD, Hill DA, O’Sullivan MJ, Dehner LP: Diagnostic gold standard for soft tissue tumours: morphology or molecular genetics? Histopathology 2000, 37:485-500 [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick SE, Garvin AJ: Recent advances in the diagnosis of pediatric soft-tissue tumors. Med Pediatr Oncol 1999, 32:373-376 [DOI] [PubMed] [Google Scholar]
- 6.Ladanyi M, Bridge JA: Contribution of molecular genetic data to the classification of sarcomas. Hum Pathol 2000, 31:532-538 [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CD, Fletcher JA, Cin PD, Ladanyi M, Woodruff JM: Diagnostic gold standard for soft tissue tumours: morphology or molecular genetics? Histopathology 2001, 39:100-103 [DOI] [PubMed] [Google Scholar]
- 8.Touriol C, Greenland C, Lamant L, Pulford K, Bernard F, Rousset T, Mason DY, Delsol G: Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like). Blood 2000, 95:3204-3207 [PubMed] [Google Scholar]
- 9.Lamant L, Dastugue N, Pulford K, Delsol G, Mariame B: A new fusion gene TPM3-ALK in anaplastic large cell lymphoma created by a (1;2)(q25;p23) translocation. Blood 1999, 93:3088-3095 [PubMed] [Google Scholar]
- 10.Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, Pinkus GS, Xiao S, Yi ES, Fletcher CD, Fletcher JA: TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 2000, 157:377-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridge JA, Kanamori M, Ma Z, Pickering D, Hill DA, Lydiatt W, Lui MY, Colleoni GW, Antonescu CR, Ladanyi M, Morris SW: Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol 2001, 159:411-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson J, Gordon T, McManus A, Mapp T, Gould S, Kelsey A, McDowell H, Pinkerton R, Shipley J, Pritchard-Jones K: Detection of the PAX3-FKHR fusion gene in paediatric rhabdomyosarcoma: a reproducible predictor of outcome? Br J Cancer 2001, 85:831-835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorensen PH, Lynch JC, Qualman SJ, Tirabosco R, Lim JF, Maurer HM, Bridge JA, Crist WM, Triche TJ, Barr FG: PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children’s oncology group. J Clin Oncol 2002, 20:2672-2679 [DOI] [PubMed] [Google Scholar]
- 14.de Alava E, Kawai A, Healey JH, Fligman I, Meyers PA, Huvos AG, Gerald WL, Jhanwar SC, Argani P, Antonescu CR, Pardo-Mindan FJ, Ginsberg J, Womer R, Lawlor ER, Wunder J, Andrulis I, Sorensen PH, Barr FG, Ladanyi M: EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing’s sarcoma. J Clin Oncol 1998, 16:1248-1255 [DOI] [PubMed] [Google Scholar]
- 15.de Alava E, Panizo A, Antonescu CR, Huvos AG, Pardo-Mindan FJ, Barr FG, Ladanyi M: Association of EWS-FLI1 type 1 fusion with lower proliferative rate in Ewing’s sarcoma. Am J Pathol 2000, 156:849-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai A, Woodruff J, Healey JH, Brennan MF, Antonescu CR, Ladanyi M: SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med 1998, 338:153-160 [DOI] [PubMed] [Google Scholar]
- 17.Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, Healey JH, Brennan MF, Bridge JA, Neff JR, Barr FG, Goldsmith JD, Brooks JS, Goldblum JR, Ali SZ, Shipley J, Cooper CS, Fisher C, Skytting B, Larsson O: Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res 2002, 62:135-140 [PubMed] [Google Scholar]
- 18.Ladanyi M, Chan WC, Triche TJ, Gerald WL: Expression profiling of human tumors: the end of surgical pathology? J Mol Diagn 2001, 3:92-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West DC, Grier HE, Swallow MM, Demetri GD, Granowetter L, Sklar J: Detection of circulating tumor cells in patients with Ewing’s sarcoma and peripheral primitive neuroectodermal tumor. J Clin Oncol 1997, 15:583-588 [DOI] [PubMed] [Google Scholar]
- 20.Kelly KM, Womer RB, Barr FG: Minimal disease detection in patients with alveolar rhabdomyosarcoma using a reverse transcriptase-polymerase chain reaction method. Cancer 1996, 78:1320-1327 [DOI] [PubMed] [Google Scholar]
- 21.Gattenloehner S, Dockhorn-Dworniczak B, Leuschner I, Vincent A, Muller-Hermelink HK, Marx A: A comparison of MyoD1 and fetal acetylcholine receptor expression in childhood tumors and normal tissues: implications for the molecular diagnosis of minimal disease in rhabdomyosarcomas. J Mol Diagn 1999, 1:23-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willeke F, Sturm JW: Minimal residual disease in soft-tissue sarcomas. Semin Surg Oncol 2001, 20:294-303 [DOI] [PubMed] [Google Scholar]
- 23.Willeke F, Mechtersheimer G, Schwarzbach M, Weitz J, Zimmer D, Lehnert T, Herfarth C, von Knebel Doeberitz M, Ridder R: Detection of SYT-SSX1/2 fusion transcripts by reverse transcriptase- polymerase chain reaction (RT-PCR) is a valuable diagnostic tool in synovial sarcoma. Eur J Cancer 1998, 34:2087-2093 [DOI] [PubMed] [Google Scholar]
- 24.Athale UH, Shurtleff SA, Jenkins JJ, Poquette CA, Tan M, Downing JR, Pappo AS: Use of reverse transcriptase polymerase chain reaction for diagnosis and staging of alveolar rhabdomyosarcoma, Ewing sarcoma family of tumors, and desmoplastic small round cell tumor. J Pediatr Hematol Oncol 2001, 23:99-104 [DOI] [PubMed] [Google Scholar]
- 25.Bagg A: Chronic myeloid leukemia: a minimalistic view of post-therapeutic monitoring. J Mol Diagn 2002, 4:1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin F, van Rhee F, Goldman JM, Cross NC: Kinetics of increasing BCR-ABL transcript numbers in chronic myeloid leukemia patients who relapse after bone marrow transplantation. Blood 1996, 87:4473-4478 [PubMed] [Google Scholar]
- 27.Dazzi F, Szydlo RM, Goldman JM: Donor lymphocyte infusions for relapse of chronic myeloid leukemia after allogeneic stem cell transplant: where we now stand. Exp Hematol 1999, 27:1477-1486 [DOI] [PubMed] [Google Scholar]
- 28.Tuveson DA, Fletcher JA: Signal transduction pathways in sarcoma as targets for therapeutic intervention. Curr Opin Oncol 2001, 13:249-255 [DOI] [PubMed] [Google Scholar]
- 29.Tomescu O, Barr FG: Chromosomal translocations in sarcomas: prospects for therapy. Trends Mol Med 2001, 7:554-559 [DOI] [PubMed] [Google Scholar]
- 30.Scappaticci FA, Marina N: New molecular targets and biological therapies in sarcomas. Cancer Treat Rev 2001, 27:317-326 [DOI] [PubMed] [Google Scholar]
- 31.Maki RG: Soft tissue sarcoma as a model disease to examine cancer immunotherapy. Curr Opin Oncol 2001, 13:270-274 [DOI] [PubMed] [Google Scholar]
- 32.Bremer C, Tung CH, Weissleder R: In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med 2001, 7:743-748 [DOI] [PubMed] [Google Scholar]
- 33.Lugo-Vicente H: Molecular biology and genetics affecting pediatric solid tumors. Bol Assoc Med P R 2000, 92:72-82 [PubMed] [Google Scholar]
- 34.Hunt KK, Feig BW: Preclinical experimental therapeutic approaches in soft tissue sarcoma. Semin Surg Oncol 1999, 17:78-82 [DOI] [PubMed] [Google Scholar]
- 35.Allander SV, Illei PB, Chen Y, Antonescu CR, Bittner M, Ladanyi M, Meltzer PS: Expression profiling of synovial sarcoma by cDNA microarrays: association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am J Pathol 2002, 161:1587-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worley BS, van den Broeke LT, Goletz TJ, Pendleton CD, Daschbach EM, Thomas EK, Marincola FM, Helman LJ, Berzofsky JA: Antigenicity of fusion proteins from sarcoma-associated chromosomal translocations. Cancer Res 2001, 61:6868-6875 [PubMed] [Google Scholar]
- 37.Antonescu CR, Tschernyavsky SJ, Woodruff JM, Jungbluth AA, Brennan MF, Ladanyi M: Molecular diagnosis of clear cell sarcoma: detection of EWS-ATF1 and MITF-M transcripts and histopathological and ultrastructural analysis of 12 cases. J Mol Diagn 2002, 4:44-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Hisaoka M, Shimajiri S, Morimitsu Y, Hashimoto H: Detection of COL1A1-PDGFB fusion transcripts in dermatofibrosarcoma protuberans by reverse transcription-polymerase chain reaction using archival formalin-fixed, paraffin-embedded tissues. Diagn Mol Pathol 1999, 8:113-119 [DOI] [PubMed] [Google Scholar]
- 39.Sheng WQ, Hisaoka M, Okamoto S, Tanaka A, Meis-Kindblom JM, Kindblom LG, Ishida T, Nojima T, Hashimoto H: Congenital-infantile fibrosarcoma: a clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin-embedded tissues. Am J Clin Pathol 2001, 115:348-355 [DOI] [PubMed] [Google Scholar]
- 40.Antonescu CR, Kawai A, Leung DH, Lonardo F, Woodruff JM, Healey JH, Ladanyi M: Strong association of SYT-SSX fusion type and morphologic epithelial differentiation in synovial sarcoma. Diagn Mol Pathol 2000, 9:1-8 [DOI] [PubMed] [Google Scholar]
- 41.Argani P, Fritsch M, Kadkol SS, Schuster A, Beckwith JB, Perlman EJ: Detection of the ETV6-NTRK3 chimeric RNA of infantile fibrosarcoma/cellular congenital mesoblastic nephroma in paraffin- embedded tissue: application to challenging pediatric renal stromal tumors. Mod Pathol 2000, 13:29-36 [DOI] [PubMed] [Google Scholar]
- 42.Chen BF, Chen ML, Liang DC, Huang YW, Liu HC, Chen SH: Detection of PAX3-FKHR and PAX7-FKHR fusion transcripts in rhabdomyosarcoma by reverse transcriptase-polymerase chain reaction using paraffin-embedded tissue. Zhonghua Yi Xue Za Zhi (Taipei) 1999, 62:86-91 [PubMed] [Google Scholar]
- 43.Okamoto S, Hisaoka M, Ishida T, Imamura T, Kanda H, Shimajiri S, Hashimoto H: Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and molecular analysis of 18 cases. Hum Pathol 2001, 32:1116-1124 [DOI] [PubMed] [Google Scholar]
- 44.Bagg A, Braziel RM, Arber DA, Bijwaard KE, Chu AY: Immunoglobulin heavy chain gene analysis in lymphomas: a multi-center study demonstrating the heterogeneity of performance of polymerase chain reaction assays. J Mol Diagn 2002, 4:81-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP: Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 1997, 22:130-131 134–138 [DOI] [PubMed] [Google Scholar]
- 46.Bijwaard KE, Fetsch JF, Przygodzki R, Taubenberger JK, Lichy JH: Detection of SYT-SSX fusion transcripts in archival synovial sarcomas by real-time reverse transcriptase-polymerase chain reaction. J Mol Diagn 2002, 4:59-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hostein I, Menard A, Bui BN, Lussan C, Wafflart J, Delattre O, Peter M, Benhattar J, Guillou L, Coindre JM: Molecular detection of the synovial sarcoma translocation t(X;18) by real-time polymerase chain reaction in paraffin-embedded material. Diagn Mol Pathol 2002, 11:16-21 [DOI] [PubMed] [Google Scholar]
- 48.Merino ME, Navid F, Christensen BL, Toretsky JA, Helman LJ, Cheung NK, Mackall CL: Immunomagnetic purging of Ewing’s sarcoma from blood and bone marrow: quantitation by real-time polymerase chain reaction. J Clin Oncol 2001, 19:3649-3659 [DOI] [PubMed] [Google Scholar]
- 49.Peter M, Gilbert E, Delattre O: A multiplex real-time PCR assay for the detection of gene fusions observed in solid tumors. Lab Invest 2001, 81:905-912 [DOI] [PubMed] [Google Scholar]
- 50.Bridge JA, Liu J, Weibolt V, Baker KS, Perry D, Kruger R, Qualman S, Barr F, Sorensen P, Triche T, Suijkerbuijk R: Novel genomic imbalances in embryonal rhabdomyosarcoma revealed by comparative genomic hybridization and fluorescence in situ hybridization: an intergroup rhabdomyosarcoma study. Genes Chromosomes Cancer 2000, 27:337-344 [DOI] [PubMed] [Google Scholar]
- 51.Bridge JA, Liu J, Qualman SJ, Suijkerbuijk R, Wenger G, Zhang J, Wan X, Baker KS, Sorensen P, Barr FG: Genomic gains and losses are similar in genetic and histologic subsets of rhabdomyosarcoma, whereas amplification predominates in embryonal with anaplasia and alveolar subtypes. Genes Chromosomes Cancer 2002, 33:310-321 [DOI] [PubMed] [Google Scholar]
- 52.Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, Pinkel D: Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science 1992, 258:818-821 [DOI] [PubMed] [Google Scholar]
- 53.Ozaki T, Paulussen M, Poremba C, Brinkschmidt C, Rerin J, Ahrens S, Hoffmann C, Hillmann A, Wai D, Schaefer KL, Boecker W, Juergens H, Winkelmann W, Dockhorn-Dworniczak B: Genetic imbalances revealed by comparative genomic hybridization in Ewing tumors. Genes Chromosomes Cancer 2001, 32:164-171 [DOI] [PubMed] [Google Scholar]
- 54.Pandita A, Zielenska M, Thorner P, Bayani J, Godbout R, Greenberg M, Squire JA: Application of comparative genomic hybridization, spectral karyotyping, and microarray analysis in the identification of subtype-specific patterns of genomic changes in rhabdomyosarcoma. Neoplasia 1999, 1:262-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skytting BT, Szymanska J, Aalto Y, Lushnikova T, Blomqvist C, Elomaa I, Larsson O, Knuutila S: Clinical importance of genomic imbalances in synovial sarcoma evaluated by comparative genomic hybridization. Cancer Genet Cytogenet 1999, 115:39-46 [DOI] [PubMed] [Google Scholar]
- 56.Tarkkanen M, Kiuru-Kuhlefelt S, Blomqvist C, Armengol G, Bohling T, Ekfors T, Virolainen M, Lindholm P, Monge O, Picci P, Knuutila S, Elomaa I: Clinical correlations of genetic changes by comparative genomic hybridization in Ewing sarcoma and related tumors. Cancer Genet Cytogenet 1999, 114:35-41 [DOI] [PubMed] [Google Scholar]
- 57.Szymanska J, Serra M, Skytting B, Larsson O, Virolainen M, Akerman M, Tarkkanen M, Huuhtanen R, Picci P, Bacchini P, Asko-Seljavaara S, Elomaa I, Knuutila S: Genetic imbalances in 67 synovial sarcomas evaluated by comparative genomic hybridization. Genes Chromosomes Cancer 1998, 23:213-219 [PubMed] [Google Scholar]
- 58.Barnard M, Bayani J, Grant R, Zielenska M, Squire J, Thorner P: Comparative genomic hybridization analysis of clear cell sarcoma of the kidney. Med Pediatr Oncol 2000, 34:113-116 [DOI] [PubMed] [Google Scholar]
- 59.Schrock E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith MA, Ning Y, Ledbetter DH, Bar-Am I, Soenksen D, Garini Y, Ried T: Multicolor spectral karyotyping of human chromosomes. Science 1996, 273:494-497 [DOI] [PubMed] [Google Scholar]
- 60.Roberts I, Gordon A, Wang R, Pritchard-Jones K, Shipley J, Coleman N: Molecular cytogenetic analysis consistently identifies translocations involving chromosomes 1, 2, and 15 in five embryonal rhabdomyosarcoma cell lines and a PAX-FOXO1A fusion gene negative alveolar rhabdomyosarcoma cell line. Cytogenet Cell Genet 2001, 95:134-142 [DOI] [PubMed] [Google Scholar]
- 61.Jalal SM, Law ME: Utility of multicolor fluorescent in situ hybridization in clinical cytogenetics. Genet Med 1999, 1:181-186 [DOI] [PubMed] [Google Scholar]
- 62.Triche TJ, Schofield D, Buckley J: DNA microarrays in pediatric cancer. Cancer J 2001, 7:2-15 [PubMed] [Google Scholar]
- 63.Wai DH, Schaefer KL, Schramm A, Korsching E, Van Valen F, Ozaki T, Boecker W, Schweigerer L, Dockhorn-Dworniczak B, Poremba C: Expression analysis of pediatric solid tumor cell lines using oligonucleotide microarrays. Int J Oncol 2002, 20:441-451 [PubMed] [Google Scholar]
- 64.Nielsen TO, West RB, Linn SC, Alter O, Knowling MA, O’Connell JX, Zhu S, Fero M, Sherlock G, Pollack JR, Brown PO, Botstein D, van de Rijn M: Molecular characterisation of soft tissue tumours: a gene expression study. Lancet 2002, 359:1301-1307 [DOI] [PubMed] [Google Scholar]
- 65.Khan J, Simon R, Bittner M, Chen Y, Leighton SB, Pohida T, Smith PD, Jiang Y, Gooden GC, Trent JM, Meltzer PS: Gene expression profiling of alveolar rhabdomyosarcoma with cDNA microarrays. Cancer Res 1998, 58:5009-5013 [PubMed] [Google Scholar]
- 66.Qualman SJ, Morotti RA: Risk assignment in pediatric soft-tissue sarcomas: an evolving molecular classification. Curr Oncol Rep 2002, 4:123-130 [DOI] [PubMed] [Google Scholar]
- 67.Biegel JA, Nycum LM, Valentine V, Barr FG, Shapiro DN: Detection of the t(2;13)(q35;q14) and PAX3-FKHR fusion in alveolar rhabdomyosarcoma by fluorescence in situ hybridization. Genes Chromosomes Cancer 1995, 12:186-192 [DOI] [PubMed] [Google Scholar]
- 68.McManus AP, O’Reilly MA, Jones KP, Gusterson BA, Mitchell CD, Pinkerton CR, Shipley JM: Interphase fluorescence in situ hybridization detection of t(2;13)(q35;q14) in alveolar rhabdomyosarcoma: a diagnostic tool in minimally invasive biopsies. J Pathol 1996, 178:410-414 [DOI] [PubMed] [Google Scholar]
- 69.Xia SJ, Pressey JG, Barr FG: Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol Ther 2002, 1:97-104 [DOI] [PubMed] [Google Scholar]
- 70.Ladanyi M: The emerging molecular genetics of sarcoma translocations. Diagn Mol Pathol 1995, 4:162-173 [DOI] [PubMed] [Google Scholar]
- 71.Barr FG, Qualman SJ, Macris MH, Melnyk N, Lawlor ER, Strzelecki DM, Triche TJ, Bridge JA, Sorensen PH: Genetic heterogeneity in the alveolar rhabdomyosarcoma subset without typical gene fusions. Cancer Res 2002, 62:4704-4710 [PubMed] [Google Scholar]
- 72.Strachan T, Read AP: PAX genes. Curr Opin Genet Dev 1994, 4:427-438 [DOI] [PubMed] [Google Scholar]
- 73.Barr FG: Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene 2001, 20:5736-5746 [DOI] [PubMed] [Google Scholar]
- 74.Barber TD, Barber MC, Tomescu O, Barr FG, Ruben S, Friedman TB: Identification of target genes regulated by PAX3 and PAX3-FKHR in embryogenesis and alveolar rhabdomyosarcoma. Genomics 2002, 79:278-284 [DOI] [PubMed] [Google Scholar]
- 75.Feinberg AP: Genomic imprinting and gene activation in cancer. Nat Genet 1993, 4:110-113 [DOI] [PubMed] [Google Scholar]
- 76.Kojima T, Asami S, Chin M, Yoshida Y, Mugishima H, Suzuki T: Detection of chimeric genes in Ewing’s sarcoma and its clinical applications. Biol Pharm Bull 2002, 25:991-994 [DOI] [PubMed] [Google Scholar]
- 77.Ladanyi M: EWS-FLI1 and Ewing’s sarcoma: recent molecular data and new insights. Cancer Biol Ther 2002, 1:330-336 [PubMed] [Google Scholar]
- 78.Udayakumar AM, Sundareshan TS, Goud TM, Devi MG, Biswas S, Appaji L, Arunakumari BS, Rajan KR, Prabhakaran PS: Cytogenetic characterization of Ewing tumors using fine needle aspiration samples: a 10-year experience and review of the literature. Cancer Genet Cytogenet 2001, 127:42-48 [DOI] [PubMed] [Google Scholar]
- 79.Sumerauer D, Vicha A, Kucerova H, Kodet R, Houskova J, Bedrnicek J, Eckschlager T: Detection of minimal bone marrow infiltration in patients with localized and metastatic Ewing sarcoma using RT-PCR. Folia Biol 2001, 47:206-210 [PubMed] [Google Scholar]
- 80.Zoubek A, Dockhorn-Dworniczak B, Delattre O, Christiansen H, Niggli F, Gatterer-Menz I, Smith TL, Jurgens H, Gadner H, Kovar H: Does expression of different EWS chimeric transcripts define clinically distinct risk groups of Ewing tumor patients? J Clin Oncol 1996, 14:1245-1251 [DOI] [PubMed] [Google Scholar]
- 81.Lin PP, Brody RI, Hamelin AC, Bradner JE, Healey JH, Ladanyi M: Differential transactivation by alternative EWS-FLI1 fusion proteins correlates with clinical heterogeneity in Ewing’s sarcoma. Cancer Res 1999, 59:1428-1432 [PubMed] [Google Scholar]
- 82.Bennicelli JL, Barr FG: Chromosomal translocations and sarcomas. Curr Opin Oncol 2002, 14:412-419 [DOI] [PubMed] [Google Scholar]
- 83.Chansky HA, Hu M, Hickstein DD, Yang L: Oncogenic TLS/ERG and EWS/Fli-1 fusion proteins inhibit RNA splicing mediated by YB-1 protein. Cancer Res 2001, 61:3586-3590 [PubMed] [Google Scholar]
- 84.Knoop LL, Baker SJ: EWS/FLI alters 5′-splice site selection. J Biol Chem 2001, 276:22317-22322 [DOI] [PubMed] [Google Scholar]
- 85.Arvand A, Welford SM, Teitell MA, Denny CT: The COOH-terminal domain of FLI-1 is necessary for full tumorigenesis and transcriptional modulation by EWS/FLI-1. Cancer Res 2001, 61:5311-5317 [PubMed] [Google Scholar]
- 86.Girnita L, Girnita A, Wang M, Meis-Kindblom JM, Kindblom LG, Larsson O: A link between basic fibroblast growth factor (bFGF) and EWS/FLI-1 in Ewing’s sarcoma cells. Oncogene 2000, 19:4298-4301 [DOI] [PubMed] [Google Scholar]
- 87.de Alava E, Antonescu CR, Panizo A, Leung D, Meyers PA, Huvos AG, Pardo-Mindan FJ, Healey JH, Ladanyi M: Prognostic impact of P53 status in Ewing sarcoma. Cancer 2000, 89:783-792 [PubMed] [Google Scholar]
- 88.Noguera R, Pellin A, Navarro S, Carda C, Llombart-Bosch A: Translocation (10;11;22)(p14;q24;q12) characterized by fluorescence in situ hybridization in a case of Ewing’s tumor. Diagn Mol Pathol 2001, 10:2-8 [DOI] [PubMed] [Google Scholar]
- 89.Mastrangelo T, Modena P, Tornielli S, Bullrich F, Testi MA, Mezzelani A, Radice P, Azzarelli A, Pilotti S, Croce CM, Pierotti MA, Sozzi G: A novel zinc finger gene is fused to EWS in small round cell tumor. Oncogene 2000, 19:3799-3804 [DOI] [PubMed] [Google Scholar]
- 90.Gerald WL, Ladanyi M, de Alava E, Cuatrecasas M, Kushner BH, LaQuaglia MP, Rosai J: Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol 1998, 16:3028-3036 [DOI] [PubMed] [Google Scholar]
- 91.Ladanyi M, Gerald W: Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res 1994, 54:2837-2840 [PubMed] [Google Scholar]
- 92.Antonescu CR, Gerald WL, Magid MS, Ladanyi M: Molecular variants of the EWS-WT1 gene fusion in desmoplastic small round cell tumor. Diagn Mol Pathol 1998, 7:24-28 [DOI] [PubMed] [Google Scholar]
- 93.Benjamin LE, Fredericks WJ, Barr FG, Rauscher FJ, III: Fusion of the EWS1 and WT1 genes as a result of the t(11;22)(p13;q12) translocation in desmoplastic small round cell tumors. Med Pediatr Oncol 1996, 27:434-439 [DOI] [PubMed] [Google Scholar]
- 94.Scharnhorst V, van der Eb AJ, Jochemsen AG: WT1 proteins: functions in growth and differentiation. Gene 2001, 273:141-161 [DOI] [PubMed] [Google Scholar]
- 95.Lee SB, Kolquist KA, Nichols K, Englert C, Maheswaran S, Ladanyi M, Gerald WL, Haber DA: The EWS-WT1 translocation product induces PDGFA in desmoplastic small round-cell tumour. Nat Genet 1997, 17:309-313 [DOI] [PubMed] [Google Scholar]
- 96.Brody RI, Ueda T, Hamelin A, Jhanwar SC, Bridge JA, Healey JH, Huvos AG, Gerald WL, Ladanyi M: Molecular analysis of the fusion of EWS to an orphan nuclear receptor gene in extraskeletal myxoid chondrosarcoma. Am J Pathol 1997, 150:1049-1058 [PMC free article] [PubMed] [Google Scholar]
- 97.Dal Cin P, Sciot R, Panagopoulos I, Aman P, Samson I, Mandahl N, Mitelman F, Van den Berghe H, Fletcher CD: Additional evidence of a variant translocation t(12;22) with EWS/CHOP fusion in myxoid liposarcoma: clinicopathological features. J Pathol 1997, 182:437-441 [DOI] [PubMed] [Google Scholar]
- 98.Barr FG: Translocations, cancer, and the puzzle of specificity. Nat Genet 1998, 19:121-124 [DOI] [PubMed] [Google Scholar]
- 99.Rabbitts TH: Perspective: chromosomal translocations can affect genes controlling gene expression and differentiation: why are these functions targeted? J Pathol 1999, 187:39-42 [DOI] [PubMed] [Google Scholar]
- 100.Sreekantaiah C, Ladanyi M, Rodriguez E, Chaganti RS: Chromosomal aberrations in soft tissue tumors: relevance to diagnosis, classification, and molecular mechanisms. Am J Pathol 1994, 144:1121-1134 [PMC free article] [PubMed] [Google Scholar]
- 101.Yang P, Hirose T, Hasegawa T, Hizawa K, Sano T: Dual-colour fluorescence in situ hybridization analysis of synovial sarcoma. J Pathol 1998, 184:7-13 [DOI] [PubMed] [Google Scholar]
- 102.Brodin B, Haslam K, Yang K, Bartolazzi A, Xie Y, Starborg M, Lundeberg J, Larsson O: Cloning and characterization of spliced fusion transcript variants of synovial sarcoma: sYT/SSX4, SYT/SSX4v, and SYT/SSX2v: possible regulatory role of the fusion gene product in wild-type SYT expression. Gene 2001, 268:173-182 [DOI] [PubMed] [Google Scholar]
- 103.Skytting B, Nilsson G, Brodin B, Xie Y, Lundeberg J, Uhlen M, Larsson O: A novel fusion gene, SYT-SSX4, in synovial sarcoma. J Natl Cancer Inst 1999, 91:974-975 [DOI] [PubMed] [Google Scholar]
- 104.Tornkvist M, Brodin B, Bartolazzi A, Larsson O: A novel type of SYT/SSX fusion: methodological and biological implications. Mod Pathol 2002, 15:679-685 [DOI] [PubMed] [Google Scholar]
- 105.Xie Y, Skytting B, Nilsson G, Gasbarri A, Haslam K, Bartolazzi A, Brodin B, Mandahl N, Larsson O: SYT-SSX is critical for cyclin D1 expression in synovial sarcoma cells: a gain of function of the t(X;18)(p11.2;q11.2) translocation. Cancer Res 2002, 62:3861-3867 [PubMed] [Google Scholar]
- 106.Nagai M, Tanaka S, Tsuda M, Endo S, Kato H, Sonobe H, Minami A, Hiraga H, Nishihara H, Sawa H, Nagashima K: Analysis of transforming activity of human synovial sarcoma-associated chimeric protein SYT-SSX1 bound to chromatin remodeling factor hBRM/hSNF2 α. Proc Natl Acad Sci USA 2001, 98:3843-3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sandberg AA, Bridge JA: Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: synovial sarcoma. Cancer Genet Cytogenet 2002, 133:1-23 [DOI] [PubMed] [Google Scholar]
- 108.Yoshida H, Nagao K, Ito H, Yamamoto K, Ushigome S: Chromosomal translocations in human soft tissue sarcomas by interphase fluorescence in situ hybridization. Pathol Int 1997, 47:222-229 [DOI] [PubMed] [Google Scholar]
- 109.Thomson B, Hawkins D, Felgenhauer J, Radich J: RT-PCR evaluation of peripheral blood, bone marrow, and peripheral blood stem cells in children and adolescents undergoing VACIME chemotherapy for Ewing’s sarcoma and alveolar rhabdomyosarcoma. Bone Marrow Transplant 1999, 24:527-533 [DOI] [PubMed] [Google Scholar]
- 110.Langezaal SM, Graadt van Roggen JF, Cleton-Jansen AM, Baelde JJ, Hogendoorn PC: Malignant melanoma is genetically distinct from clear cell sarcoma of tendons and aponeurosis (malignant melanoma of soft parts). Br J Cancer 2001, 84:535-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gill S, McManus AP, Crew AJ, Benjamin H, Sheer D, Gusterson BA, Pinkerton CR, Patel K, Cooper CS, Shipley JM: Fusion of the EWS gene to a DNA segment from 9q22–31 in a human myxoid chondrosarcoma. Genes Chromosomes Cancer 1995, 12:307-310 [DOI] [PubMed] [Google Scholar]
- 112.Sjogren H, Wedell B, Meis-Kindblom JM, Kindblom LG, Stenman G, Kindblom JM: Fusion of the NH2-terminal domain of the basic helix-loop-helix protein TCF12 to TEC in extraskeletal myxoid chondrosarcoma with translocation t(9;15)(q22;q21). Cancer Res 2000, 60:6832-6835 [PubMed] [Google Scholar]
- 113.Attwooll C, Tariq M, Harris M, Coyne JD, Telford N, Varley JM: Identification of a novel fusion gene involving hTAFII68 and CHN from a t(9;17)(q22;q11.2) translocation in an extraskeletal myxoid chondrosarcoma. Oncogene 1999, 18:7599-7601 [DOI] [PubMed] [Google Scholar]
- 114.Naumann S, Krallman PA, Unni KK, Fidler ME, Neff JR, Bridge JA: Translocation der(13;21)(q10;q10) in skeletal and extraskeletal mesenchymal chondrosarcoma. Mod Pathol 2002, 15:572-576 [DOI] [PubMed] [Google Scholar]
- 115.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH: A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet 1998, 18:184-187 [DOI] [PubMed] [Google Scholar]
- 116.Cools J, Wlodarska I, Somers R, Mentens N, Pedeutour F, Maes B, De Wolf-Peeters C, Pauwels P, Hagemeijer A, Marynen P: Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer 2002, 34:354-362 [DOI] [PubMed] [Google Scholar]
- 117.Antonescu CR, Tschernyavsky SJ, Decuseara R, Leung DH, Woodruff JM, Brennan MF, Bridge JA, Neff JR, Goldblum JR, Ladanyi M: Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res 2001, 7:3977-3987 [PubMed] [Google Scholar]
- 118.Nilsson M, Hoglund M, Panagopoulos I, Sciot R, Dal Cin P, Debiec-Rychter M, Mertens F, Mandahl N: Molecular cytogenetic mapping of recurrent chromosomal breakpoints in tenosynovial giant cell tumors. Virchows Arch 2002, 441:475-480 [DOI] [PubMed] [Google Scholar]
- 119.Hibbard MK, Kozakewich HP, Dal Cin P, Sciot R, Tan X, Xiao S, Fletcher JA: PLAG1 fusion oncogenes in lipoblastoma. Cancer Res 2000, 60:4869-4872 [PubMed] [Google Scholar]
- 120.Gisselsson D, Hibbard MK, Dal Cin P, Sciot R, Hsi BL, Kozakewich HP, Fletcher JA: PLAG1 alterations in lipoblastoma: involvement in varied mesenchymal cell types and evidence for alternative oncogenic mechanisms. Am J Pathol 2001, 159:955-962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sozzi G, Minoletti F, Miozzo M, Sard L, Musso K, Azzarelli A, Pierotti MA, Pilotti S: Relevance of cytogenetic and fluorescent in situ hybridization analyses in the clinical assessment of soft tissue sarcoma. Hum Pathol 1997, 28:134-142 [DOI] [PubMed] [Google Scholar]
- 122.Pedeutour F, Simon MP, Minoletti F, Barcelo G, Terrier-Lacombe MJ, Combemale P, Sozzi G, Ayraud N, Turc-Carel C: Translocation, t(17;22)(q22;q13), in dermatofibrosarcoma protuberans: a new tumor-associated chromosome rearrangement. Cytogenet Cell Genet 1996, 72:171-174 [DOI] [PubMed] [Google Scholar]
- 123.O’Brien KP, Seroussi E, Dal Cin P, Sciot R, Mandahl N, Fletcher JA, Turc-Carel C, Dumanski JP: Various regions within the α-helical domain of the COL1A1 gene are fused to the second exon of the PDGFB gene in dermatofibrosarcomas and giant-cell fibroblastomas. Genes Chromosomes Cancer 1998, 23:187-193 [PubMed] [Google Scholar]
- 124.Qi H, Dal Cin P, Hernandez JM, Garcia JL, Sciot R, Fletcher C, Van Eyken P, De Wever I, Van den Berghe H: Trisomies 8 and 20 in desmoid tumors. Cancer Genet Cytogenet 1996, 92:147-149 [DOI] [PubMed] [Google Scholar]
- 125.Ladanyi M, Lui MY, Antonescu CR, Krause-Boehm A, Meindl A, Argani P, Healey JH, Ueda T, Yoshikawa H, Meloni-Ehrig A, Sorensen PH, Mertens F, Mandahl N, van den Berghe H, Sciot R, Cin PD, Bridge J: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 2001, 20:48-57 [DOI] [PubMed] [Google Scholar]
- 126.Maire G, Martin L, Michalak-Provost S, Gattas GJ, Turc-Carel C, Lorette G, Pedeutour F: Fusion of COL1A1 exon 29 with PDGFB exon 2 in a der(22)t(17;22) in a pediatric giant cell fibroblastoma with a pigmented Bednar tumor component: evidence for age-related chromosomal pattern in dermatofibrosarcoma protuberans and related tumors. Cancer Genet Cytogenet 2002, 134:156-161 [DOI] [PubMed] [Google Scholar]
- 127.Dehner LP: Some general considerations about the clinicopathologic aspects of soft tissue tumors. Coffin MC Dehner LP O’Shea PA eds. Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. 1997:1-14 Williams & Wilkins Philadelphia
- 128. Fletcher CDM UK Mertens F eds. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Soft Tissue and Bone. 2002. IARC Press Lyon