Abstract
Her-2/neu, a proto-oncogene located on chromosome 17, is an important biomarker in breast carcinoma. Immunohistochemistry (IHC) is currently the most widely used method for assessing Her-2/neu status. Some IHC-positive cases do not show Her-2/neu gene amplification by fluorescence in situ hybridization (FISH). It has been suggested that some of these IHC “false positive” results may in part be due to increased copy number of chromosome 17 resulting in increased Her-2/neu protein expression. We analyzed IHC and FISH data from 561 cases of invasive breast carcinoma to test this hypothesis. IHC and FISH for Her-2/neu were performed on formalin-fixed, paraffin-embedded sections of 561 invasive breast carcinomas. The IHC results were interpreted as 0, 1+, 2+, or 3+ according to the manufacturer’s recommended criteria. The FISH results were expressed as a ratio of Her-2/neu/chromosome 17 and were interpreted as positive (> = 2.0) or negative (<2.0) for gene amplification according to the manufacturer’s recommended scoring system. We found that in IHC 3+/FISH-negative cases (n = 15) both the average chromosome 17 copy number and the average Her-2/neu copy number were significantly higher than that in IHC (0 to 2+)/FISH-negative cases (n = 411) (2.45 vs. 1.68; P < 0.0001, and 3.19 vs. 1.95; P < 0.0001, respectively). In contrast, the IHC 2+/FISH-negative cases did not exhibit a significantly increased number of chromosome 17 compared to IHC 0 to 1+ cases. In addition, the average copy number of chromosome 17 in FISH-positive cases (n = 135) was significantly higher than that in FISH-negative cases (n = 426) (2.27 vs. 1.70; P < 0.0001), indicating a general association of increased chromosome 17 copy number with Her-2/neu gene amplification. Thus, our data suggest that IHC 3+ immunostaining without scorable gene amplification may indeed be, at least in some cases, the result of increased Her-2/neu protein expression secondary to an increased copy number of chromosome 17, associated with an increased total number of Her-2/neu gene copies per tumor cell.
Her-2/neu (also known as c-erbB2 or ERBB2) is a proto-oncogene located on chromosome 17. It encodes a transmembrane growth factor receptor with tyrosine kinase activity. Overexpression and/or amplification of Her-2/neu is detected in approximately 20% to 30% of invasive ductal carcinomas of the breast. 1, 2, 3, 4 It has been well documented that overexpression and/or amplification of Her-2/neu is associated with poor prognosis. 1, 5, 6, 7 Patients with increased expression of Her-2/neu also show a poorer response to non-anthracycline containing cytotoxic 8, 9, 10, 11 and hormonal 12, 13, 14 therapies. With the introduction of Herceptin, a recombinant “humanized” monoclonal antibody against Her-2/neu for treatment of metastatic breast cancer, the accurate assessment of Her-2/neu status has become essential in determining the clinical management of breast cancer patients.
Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) have emerged as the two most widely used methods to evaluate Her2/neu status in breast cancer. Numerous studies have shown that the overexpression of Her-2/neu protein is closely correlated with amplification of Her-2/neu gene in the IHC 3+ cases, but not in the IHC 2+ cases. 4, 5, 15, 16, 17 The positive Her-2/neu immunostains with no evidence of Her-2/neu gene amplification have sometimes been considered “false positive,” particularly in the IHC 2+ cases. Chromosomal aneusomy affecting chromosome 17 occurs in breast carcinomas and may complicate the scoring of Her-2/neu amplification. 18, 19 In this study, we sought to address the issue of chromosome 17 polysomy as a contributing factor to strong positive Her-2/neu immunostaining in the absence of Her-2/neu amplification as scored by dual-color FISH.
Materials and Methods
Case Selection
Among cases received for Her-2/neu testing, all cases of invasive breast carcinoma during a 5-month period and additional IHC 3+ cases during the following 8 months were included in this study. There were a total of 561 cases. All IHC and FISH tests were performed in the Department of Pathology at Memorial Sloan-Kettering Cancer Center.
Immunohistochemistry and FISH
Serial sections of 4- to 5-μm thick were used for both IHC and FISH tests. IHC was performed using HercepTest kit (DAKO, Carpinteria, CA) and the results were interpreted as negative (0 or 1+) or positive (2+ or 3+) according to the manufacturer’s recommended scoring system.
The PathVysion Her-2/neu probe kit (Vysis Inc, Downers Grove, IL) was used for the FISH analysis. In brief, the sections were baked overnight at 56°C, and the invasive carcinoma components were located on a corresponding H&E stained section. Unstained sections were deparaffinized in CitriSolv (Vysis Inc, Downers Grove, IL), dehydrated in 100% ethanol, and air-dried. Slides were then subjected to protease digestion for 45 to 60 minutes, denatured, and hybridized with pre-warmed probes (Her2/neu/CEP17 SG probe; Vysis) overnight at 37°C. They were then washed with post-hybridization wash buffer at 72°C and counterstained with DAPI, mounted, and stored in dark before signal enumeration. Slides were first scanned at low power using a DAPI filter to identify areas of optimal tissue digestion and non-overlapping nuclei. The number of chromosome 17 signals, Her-2/neu signals, and the number of tumor nuclei scored were recorded for each case. Cases were interpreted as amplified when the ratio of Her-2/chromosome17 signals was equal or greater than 2.0.
Statistics
The statistical analyses were performed using the Mann-Whitney test. All reported P values are two-tailed.
Results
The data are summarized in Table 1 . Among the 561 tumors tested, Her-2/neu was negative by both IHC (0 or 1+) and FISH methods in 368 tumors (Table 1) . The average copy number of chromosome 17 in these tumors was 1.67 per tumor cell. This value of less than 2.0 copies per cell reflects the partial loss of nuclear material due to truncation of the tumor nuclei in the 4- to 5-μm thick sections used for the tests since breast carcinoma nuclei are generally greater than 5 μm in diameter.
Table 1.
Summary Data on Average Chromosome 17 Copy Number and Her-2/neu Gene Copies per Tumor Nucleus
| IHC | FISH negative (Her-2/chr 17 ratio < 2.0) | FISH positive (Her-2/chr 17 ratio ≥ 2.0) | ||||
|---|---|---|---|---|---|---|
| N | Chr 17 copy number (range) | Her-2 copy number (range) | N | Chr 17 copy number (range) | Her-2 copy number (range) | |
| 0 | 202 | 1.67 (1.03–4.28) | 1.87 (1.04–4.71) | 2 | 1.60 (1.44–1.77) | 6.46 (4.25–8.67) |
| 1+ | 166 | 1.67 (1.15–3.20) | 1.98 (1.10–4.94) | 4 | 2.57 (1.96–3.44) | 9.21 (3.92–14.1) |
| 2+ | 43 | 1.75 (1.00–3.00) | 2.19 (1.00–3.86) | 20 | 1.95 (1.03–3.38) | 6.87 (2.07–20.4) |
| 3+ | 15 | 2.45 (1.85–3.69) | 3.19 (1.85–5.21) | 109 | 2.33 (1.07–4.96) | 11.0 (3.21–40.7) |
| Total | 426 | 1.70 (1.00–4.28) | 135 | 2.27 (1.03–4.96) | ||
N, number of cases.
There were a total of 58 cases that were Her-2/neu positive by IHC (43 cases of IHC 2+ and 15 cases of IHC 3+), but negative by FISH (Table 1) . The average copy number of chromosome 17 in IHC 3+/FISH-negative cases (n = 15) (Figure 1) was significantly higher than that in IHC (0 to 2+)/FISH-negative cases (n = 411) (2.45 vs. 1.68; P < 0.0001), whereas the IHC 2+/FISH-negative cases did not exhibit a significantly increased copy number of chromosome 17 in comparison to the IHC-negative/FISH-negative cases (1.75 vs. 1.67).
Figure 1.
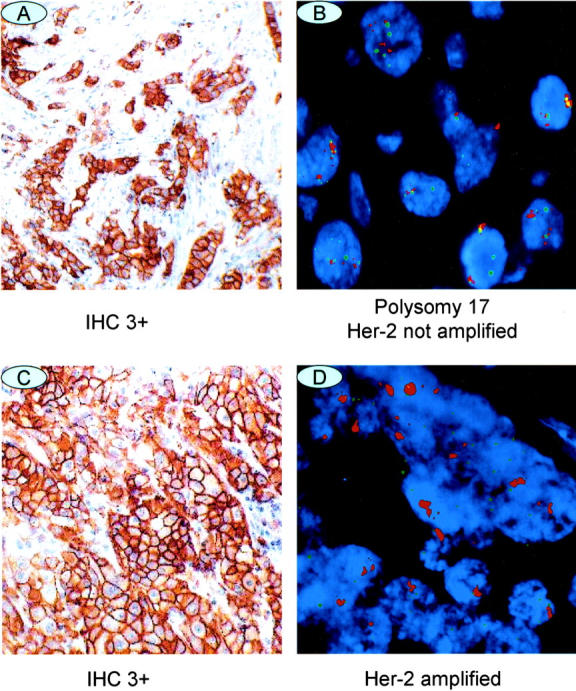
Representative examples of IHC (×10) and FISH (×100) images of Her-2/neu. A and B: A case of IHC 3+/FISH-negative. C and D: A case of IHC 3+ and FISH-positive.
The data also demonstrated that the average chromosome 17 copy number in FISH-positive tumors (n = 135) overall was significantly higher than that in FISH-negative tumors (n = 426) (2.27 vs. 1.70; P < 0.0001), indicating that polysomy 17 was more commonly seen in tumors with Her-2/neu gene amplification.
When the data were analyzed based on the absolute or unadjusted Her-2/neu signals, ie, Her-2/neu gene copies, per tumor nucleus, the impact of polysomy 17 could be clearly seen (Table 1) . The average Her-2/neu gene copy number in IHC 3+/FISH-negative tumors (n = 15) was significantly higher than that in IHC 0 to 2+/FISH-negative tumors (n = 411) (3.19 vs. 1.95; P < 0.0001). The detailed data on these 15 tumors are presented in Table 2 .
Table 2.
FISH data on 15 Tumors with IHC 3+/FISH-Negative Results
| Case number | FISH ratio | Chrosome 17 copy number | Her-2/neu gene copy number |
|---|---|---|---|
| 1 | 1 | 1.85 | 1.85 |
| 2 | 1 | 1.96 | 1.89 |
| 3 | 1 | 2.18 | 2.21 |
| 4 | 1 | 2.32 | 2.42 |
| 5 | 1 | 2.57 | 2.57 |
| 6 | 1.2 | 2.32 | 2.72 |
| 7 | 1.5 | 1.93 | 2.98 |
| 8 | 1.3 | 2.31 | 3.03 |
| 9 | 1.2 | 2.72 | 3.34 |
| 10 | 1.7 | 1.96 | 3.27 |
| 11 | 1.4 | 2.54 | 3.50 |
| 12 | 1.6 | 2.52 | 3.91 |
| 13 | 1.1 | 3.69 | 4.17 |
| 14 | 1.7 | 2.86 | 4.75 |
| 15 | 1.7 | 3.07 | 5.30 |
Discussion
Studies have shown that occasional breast carcinomas with Her-2/neu IHC 3+ immunostaining may be negative for gene amplification by FISH according to standard scoring criteria. 15, 17, 20 The FISH method is technically more standardized and less affected by tissue variables than the IHC method, and has emerged as the gold standard for assessment of Her-2/neu gene amplification status. The findings of IHC 3+ staining without gene amplification has been generally attributed to two factors: false positive immunostaining or protein overexpression without gene amplification. Our data provide the evidence for a third factor, namely chromosome 17 aneuploidy or polysomy.
Our data show that tumors with Her-2/neu gene amplification had significantly more copies of chromosome 17 than tumors without Her-2/neu gene amplification. This may reflect the fact that both DNA aneuploidy and Her-2/neu gene amplification tend to occur more frequently in poorly differentiated high-grade carcinomas. 19, 21, 22 Polysomy 17 in these tumors would have an additive effect with genuine Her-2/neu amplification and contribute to the overall increase of Her-2/neu gene copies in the tumor cells. Similarly but less dramatically, polysomy 17 in the absence of Her-2/neu gene amplification would result in a modest increase of Her-2/neu gene copies in the tumor cells. This modest but significant increase as demonstrated in this study in the Her-2/neu gene copies may result, in some cases, in an increased Her-2/neu protein production to the level that could be demonstrated by IHC staining as strong as 3+.
It should be noted that the average chromosome 17 copy number per nucleus for each tumor varied from 1.00 to 4.28 among FISH-negative tumors and from 1.03 to 4.96 among FISH-positive tumors in this study. The value of equal or close to 1.0 may be the result of nuclear truncation or true loss of chromosome 17 in the tumor cells. 19, 21 This brings out a technical issue of the FISH assay. A positive result in a dual-color FISH assay is defined as a ratio of Her-2/neu signal/chromosome 17 signal equal or greater than 2.0. A positive result in a single-color FISH assay, where only the Her-2/neu signal is detected, is usually defined as a number of Her-2/neu signals equal or greater than 4.0. Thus, there will be cases that are FISH negative using the dual-color system, but positive in the single-color system due to polysomy 17. Vice versa, positive cases in the dual-color assay could be negative in the single-color assay due to nuclear truncation or chromosome 17 monosomy. These discrepant results usually occur in tumors of borderline Her-2/neu amplification, whose clinical response to Herceptin-based therapy remains to be systematically studied. In addition, Grushko et al 23 have shown that borderline/low-level Her-2/neu amplification is more often seen in BRCA1-associated than in sporadic breast cancers. The overall frequency of borderline/low-level Her-2/neu amplification (ratio of 1.7 to 2.5) in the current study was 8.5%, which is in line with the reported frequency for sporadic tumors. The frequency of polysomy 17 in this subset of tumors did not differ from the entire group, that is, an increased chromosome 17 copy number was observed in IHC 3+ cases, but not in IHC 0 to 2+ cases.
The finding that the IHC 2+/FISH-negative tumors were not associated with chromosome 17 polysomy suggests that this group of tumors may behave more similarly to the IHC-negative (0 to 1+)/FISH-negative tumors than to the IHC 3+/FISH-negative tumors and may be truly Her-2/neu negative in a biological and clinical sense.
We also identified tumors with increased copy number of chromosome 17 or even Her-2/neu amplification that showed no Her-2/neu immunostaining, ie, IHC 0. Conversely, not all tumors in the IHC 3+/FISH-negative group showed markedly increased chromosome 17 copy number. Thus, while the increased chromosome 17 copy number in tumors without Her-2/neu amplification is statistically associated with “false positive” IHC 3+ staining, it is not strictly sufficient or absolutely necessary for the IHC 3+ staining in these tumors.
The important issues of biological behavior and therapeutic response of this subset of tumors remain to be addressed. Since, as our data demonstrated, the average chromosome 17 copy number in FISH-positive tumors, regardless of IHC status, was significantly higher than that in FISH-negative tumors, and increased number of chromosome 17 and Her-2/neu gene copy was statistically associated with the strong IHC (3+) staining, it will be reasonable to examine whether these IHC 3+/FISH-negative tumors with polysomy 17 are biologically distinct from other FISH-negative tumors and more similar to tumors with conventional Her-2/neu amplification, especially in terms of their response to Herceptin-based therapy.
Address reprint requests to Beiyun Chen, M.D., Ph.D., Department of Pathology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: chenb@mskcc.org.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL: Human breast cancer: correlation of relapse and survival with amplification of the Her-2/neu oncogene. Science 1987, 235:177-181 [DOI] [PubMed] [Google Scholar]
- 2.Pauletti G, Dandekar S, Rong HM, Ramos L, Peng H, Seshadri R, Slamon DJ: Assessment of methods for tissue-based detection of the Her-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 2000, 18:3651-3664 [DOI] [PubMed] [Google Scholar]
- 3.Pauletti G, Godolphin W, Press MF, Slamon DJ: Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996, 13:63-72 [PubMed] [Google Scholar]
- 4.Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ: Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol 1999, 17:1974-1982 [DOI] [PubMed] [Google Scholar]
- 5.Kaptain S, Tan LK, Chen B: Her-2/neu and breast cancer. Diagn Mol Pathol 2001, 10:139-152 [DOI] [PubMed] [Google Scholar]
- 6.Ravdin PM, Chamness GC: The c-erbB-2 proto-oncogene as a prognostic and predictive marker in breast cancer: a paradigm for the development of other macromolecular markers - a review. Gene 1995, 159:19-27 [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A: Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244:707-712 [DOI] [PubMed] [Google Scholar]
- 8.Menard S, Valagussa P, Pilotti S, Gianni L, Biganzoli E, Boracchi P, Tomasic G, Casalini P, Marubini E, Colnaghi MI, Cascinelli N, Bonadonna G: Response to cyclophosphamide, methotrexate, and fluorouracil in lymph node-positive breast cancer according to HER2 overexpression and other tumor biologic variables. J Clin Oncol 2001, 19:329-335 [DOI] [PubMed] [Google Scholar]
- 9.Pegram MD, Pauletti G, Slamon DJ: Her-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res 1998, 52:65-77 [DOI] [PubMed] [Google Scholar]
- 10.Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, Cirrincione CT, Budman DR, Wood WC, Barcos M: C-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med 1994, 330:1260-1266 [DOI] [PubMed] [Google Scholar]
- 11.Thor AD, Berry DA, Budman DR, Muss HB, Kute T, Henderson IC, Barcos M, Cirrincione C, Edgerton S, Allred C, Norton L, Liu ET: C-erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst 1998, 90:1346-1360 [DOI] [PubMed] [Google Scholar]
- 12.De Placido S, Carlomagno C, De Laurentiis M, Bianco AR: C-erbB2 expression predicts tamoxifen efficacy in breast cancer patients. Breast Cancer Res Treat 1998, 52:55-64 [DOI] [PubMed] [Google Scholar]
- 13.Ross JS, Fletcher JA: The Her-2/neu oncogene: prognostic factor, predictive factor, and target for therapy. Semin Cancer Biol 1999, 9:125-138 [DOI] [PubMed] [Google Scholar]
- 14.Borg A, Baldetorp B, Fernö M, Killander D, Olsson H, Rydén, Sigurdsson H: ERBB2 amplification is associated with tamoxifen resistance in steroid-receptor-positive breast cancer. Cancer Lett 1994, 81:137-144 [DOI] [PubMed] [Google Scholar]
- 15.Mass R, Sanders C, Kasian C, Everett T, Johnson L: The concordance between the clinical trials assay (CTA) and fluorescence in situ hybridization (FISH) in the Herceptin pivotal trials. Proceedings of American Society of Clinical Oncology 2000, 19:76a(Abstract) [Google Scholar]
- 16.Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ: Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol 1999, 17:1983-1987 [DOI] [PubMed] [Google Scholar]
- 17.Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM: Discrepancies in laboratory testing of eligibility for Trastuzumab therapy: apparent immunohistochemical false-positives do not get the message. J Clin Oncol 2001, 19:2714-2721 [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Saboorian MH, Frenkel EP, Haley BB, Siddiqui MT, Gokaslan S, Hynan L, Ashfaq R: Aneusomy 17 in breast cancer: its role in HER-2/neu protein expression and implication for clinical assessment of HER-2/neu status. Mod Pathol 2002, 15:137-145 [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto F, Miyoshi Y, Egawa C, Kasugai T, Takami S, Inazawa J, Noguchi S: Clinicopathologic analysis of breast carcinoma with chromosomal aneusomy detected by fluorescence in situ hybridization. Cancer 2001, 93:165-170 [DOI] [PubMed] [Google Scholar]
- 20.Birner P, Oberhuber G, Stani J, Reithofer C, Samonigg H, Hausmaninger H, Kubista E, Kwasny W, Kandioler-Eckersberger D, Gnant M, Jakesz R, : The Austrian Breast & Colorectal Cancer Study Group: evaluation of the United States Food and Drug Administration approved scoring and test system of Her-2 protein expression in breast cancer. Clin Cancer Res 2001, 7:1669-1675 [PubMed] [Google Scholar]
- 21.Nakopoulou L, Giannopoulou I, Trafalis D, Gakiopoulou H, Keramopoulos A, Davaris P: Evaluation of numeric alterations of chromosomes 1 and 17 by in situ hybridization in invasive breast carcinoma with clinicopathologic parameters. Appl Immunohistochem Mol Morphol 2002, 10:20-28 [DOI] [PubMed] [Google Scholar]
- 22.Hoff ER, Tubbs RR, Myles JL, Procop GW: HER2/neu amplification in breast cancer: stratification by tumor type and grade. Am J Clin Pathol 2002, 117:916-921 [DOI] [PubMed] [Google Scholar]
- 23.Grushko TA, Blackwood MA, Schumm PL, Hagos FG, Adeyanju MO, Feldman MD, Sanders MO, Weber BL, Olopade OI: Molecular-cytogenetic analysis of HER-2/neu gene in BRCA1-associated breast cancers. Cancer Res 2002, 62:1481-1488 [PubMed] [Google Scholar]


