Abstract
In myxoid/round cell liposarcoma, the t(12;16)(q13;p11) and its associated fusion transcript, FUS-CHOP, characterize greater than 95% of cases. The variant translocation t(12;22)(q13;q12) and associated EWS-CHOP fusion transcript are rare. A second non-random aberration observed in roughly 20% of Ewing’s sarcomas, and to a lesser extent other select sarcomas, is the unbalanced 1;16 translocation. Recognition of this secondary aberration in the absence of an obvious primary karyotypic abnormality strongly suggests that the use of other genetic approaches will be informative in uncovering a clinically suspected primary anomaly. The following case illustrates the utility of molecular cytogenetic and reverse transcriptase-polymerase chain reaction techniques in diagnosing an ins(22;12)(q12;q13q14) and associated EWS-CHOP fusion transcript in a myxoid/round cell liposarcoma exhibiting a der(16)t(1;16)(q11;q11).
Case Reports
A 36-year-old male presented with a 2-year history of a painless left calf mass. The patient, employed as a truck driver, believed the swelling of his lower leg was related to using the clutch on his vehicle. Physical examination revealed a firm, non-tender mass in the left lower leg that was approximately 10 cm in longitudinal dimension. Despite the extensive size, no neurological or vascular compromise was identified. Magnetic resonance imaging (MRI) demonstrated a non-homogenous mass, arising in the left lateral compartment and encasing the fibula. Technecium-99 scanning showed intense signal activity emanating from within the mass, but was negative for metastatic disease. An open biopsy was performed.
Microscopically, the biopsy specimen was composed of uni- and multi-vacuolar lipoblasts embedded in a myxoid matrix with a delicate plexiform capillary vascular network consistent with a diagnosis of myxoid liposarcoma. Round cell differentiation was not identified within the biopsy specimen. Subsequently, a radical segmental resection including a left fibulectomy was performed. Grossly, a lobulated gelatinous mass measuring 18 × 6 × 6 cm entrapping the fibula was seen. In contrast to the open biopsy, histological evidence of focal round cell differentiation (approximately 20%) was evident in the subsequent resection specimen. Four months following the surgical resection, the patient remains disease-free.
Cytogenetic Analysis
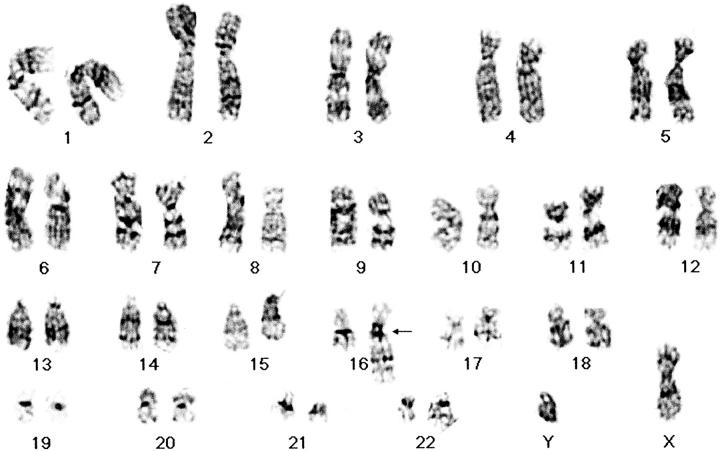
Cytogenetic analysis was performed on sterile, representative samples of both the biopsy and resection specimens using previously described culture and harvest techniques. 1 Analysis of 44 GTG-banded metaphase cells (from both specimens) revealed a clearly abnormal chromosome 16 [der(16)t(1;16)(q11;q11)] and a possible narrowing of the dark bands in region q13–21 on chromosome 12 (Figure 1) .
Figure 1.
Representative GTG-banded karyotype illustrating the abnormal chromosome 16 (arrow).
Molecular Cytogenetic Analysis
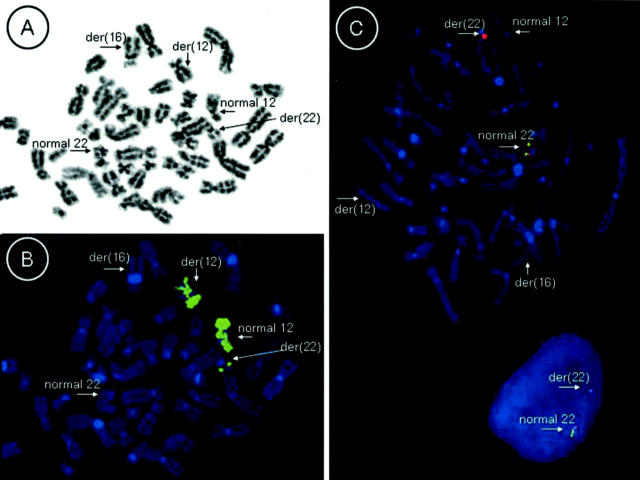
In an effort to determine whether an underlying anomaly of chromosome 12 was present, fluorescence in situ hybridization (FISH) studies were performed on de-stained metaphase preparations with a Spectrum Green-labeled chromosome 12-specific paint probe (Vysis, Inc., Downers Grove, IL) using previously described methods. 1 This analysis revealed that a small portion of chromosome 12 was inserted into the long arm of chromosome 22 (Figure 2 A and B) . These findings, in conjunction with the clinicopathologically favored diagnosis of myxoid/round cell liposarcoma, led us to examine the EWS gene locus using bicolor FISH techniques with 22q12 (EWS) breakpoint flanking probes as previously described with minor modifications. 2 Briefly, a probe mix containing Spectrum Orange-labeled G9 and Spectrum Green-labeled F7 cosmid probes 3 (Vysis, Inc. Translation Kit) and LSI buffer (50% formamide/2X SSC, Vysis, Inc.) was applied to a de-stained metaphase cell slide preparation and sealed under a glass coverslip. The cells and probes were co-denatured at 75°C for 1 minute on a HYBrite instrument (Vysis, Inc.) and subsequently incubated overnight at 37°C. After a 2-minute post-wash in 0.4X SSC at 72°C, the slide was rinsed in 2X SSC/1%NP-40 at room temperature and counterstained with 4,6-diamino-2-phenole-indole (DAPI). Failure to detect the probe signal distal to the 22q12 breakpoint on the der(22) suggests a disruption of the EWS locus (Figure 2C) . The following nomenclature reflects the combined cytogenetic and FISH findings: 46,XY,del(12)(q13q14),der(16)t(1;16)(q11;q11),der(22)ins(22;12)(q12;q13q14)del(22)(q12q12). ish der(22)(wcp12+,G9+,F7−).
Figure 2.
A and B: Whole chromosome 12 paint probe FISH performed on metaphase cells reveals an insertion of chromosome 12 material on the long arm of one chromosome 22 homologue. (A and B represent inverted DAPI and FISH images respectively.) C: Bicolor FISH studies performed on metaphase and interphase cells with an EWS (22q12) breakpoint flanking probe set reveals loss of the probe signal just distal to the breakpoint on the derivative chromosome 22 indicating a disruption of the EWS locus.
Reverse Transcriptase-Polymerase Chain Reaction Analysis for FUS-CHOP and EWS-CHOP Fusion Transcripts
Total RNA was extracted from lesional tissue using the guanidinium isothiocyanate-phenol chloroform method and RNA Wiz reagent (Ambion, Inc, Austin, TX). Three micrograms of total RNA were subjected to reverse transcriptase-polymerase chain reaction (RT-PCR) using the Qiagen 1 Step RT-PCR kit (Qiagen, Inc., Valencia, CA) and a forward primer either within exon 5 of FUS (5[prime]-CAG CCA GCA GCC TAG CTA TG-[prime]3) or within exon 7 of EWS (5[prime]-CTG GAT CCT ACA GCC AAG CTC CAA G-[prime]3) and a reverse primer within exon 3 of CHOP (5[prime]-TGT CCC GAA GGA GAA AGG CAA TG-[prime]3). The RT-PCR conditions used have been described previously. 4 Direct automated sequencing of the RT-PCR product, identified by agarose gel electrophoresis, revealed a 179-bp product with an in-frame junction of exon 7 of EWS to exon 2 of CHOP (Figure 3) . This finding is identical to previously detailed cases with the EWS-CHOP fusion transcript. 4
Figure 3.
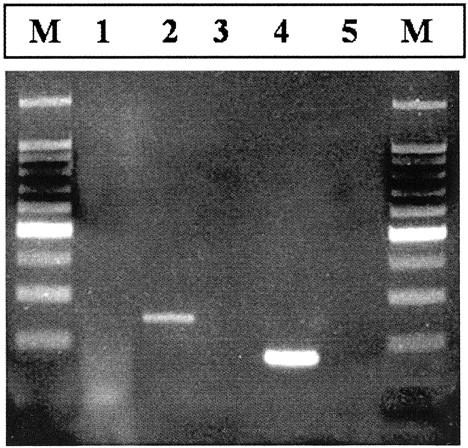
Ethidium-stained gel of the RT-PCR results. Total RNA was extracted from frozen tumor tissue and subjected to RT-PCR using FUS or EWS and CHOP primers (see Case Report for primer sequences). Lane 1, no product is identified in the patient sample using TLS-CHOP primers; lane 2, TLS-CHOP fusion transcript positive control (250 bp); lane 3, negative control (no RNA); lane 4, patient sample exhibiting an EWS-CHOP fusion transcript (179 bp); lane 5, negative control (no reverse transcriptase); and M, a DNA molecular weight marker (100 bp ladder).
Discussion
The differential diagnosis of myxoid liposarcoma is extensive and includes lipoblastoma, well-differentiated liposarcoma with extensive myxoid change, myxoid malignant fibrous histiocytoma, and myxoid chondrosarcoma, and is further confounded when round cell differentiation is identified. 5 Cytogenetic analysis is a powerful diagnostic adjunct in the classification of soft tissue sarcomas and has been shown to be of specific value in subclassifying histologically borderline or difficult adipose tissue tumors. 6, 7, 8 Typically, a distinct advantage of cytogenetic analysis (in contrast to other genetic approaches such as FISH and RT-PCR) is that it provides a global assessment of both primary and secondary abnormalities in a specific neoplasm. Rarely though, the characteristic primary abnormality may be masked as an unusual translocation variant, a more complex rearrangement, or as an inconspicuous or submicroscopic anomaly. In the current case, the detection of the secondary structural abnormality, der(16)t(1;16) alerted us that an underlying primary abnormality was likely present.
The unbalanced translocation der(16)t(1;16) has been detected by cytogenetic analysis in both hematological and non-hematologic malignancies, including breast cancer, Wilms’ tumor, retinoblastoma, Ewing’s sarcoma, alveolar rhabdomyosarcoma, and extraskeletal myxoid chondrosarcoma. 9, 10, 11 The variability of the 1q and 16q breakpoints indicates that the important consequence of this chromosomal aberration is the imbalance of the long arms of chromosomes 1 and 16, leading to a relative gain of 1q and loss of 16q material, rather than the break of the two chromosomes. 9 It has been proposed that this anomaly may represent an early event in clonal evolution and may portend an adverse prognosis. 9, 12, 13, 14, 15 A single case of myxoid liposarcoma with a 12;16 translocation (FUS-CHOP) has reportedly exhibited a der(16)t(1;16) 16 (we have also encountered such a case, unpublished observation), however, to the best of our knowledge, the der(16)t(1;16) has never been described in an EWS-CHOP fusion transcript positive myxoid/round cell liposarcoma.
Without the presence of the der(16)t(1;16) the subtle rearrangement involving chromosomes 12 and 22 would have likely been overlooked and the specimen interpreted as karyotypically normal (false negative). The chromosomal event of insertion (as seen in the current case) has not yet been described in association with the EWS-CHOP fusion transcript. Less than 5% of myxoid/round cell liposarcomas are characterized by an EWS-CHOP fusion transcript. 17, 18, 19 The orientation of EWS in relation to CHOP may explain this low frequency. The normal EWS gene is transcribed from centromere to telomere while the transcription of CHOP proceeds in the opposite direction. Hence, the formation of the EWS-CHOP fusion transcript is possible only if another genomic aberration, such as an inversion, occurs in addition to the chromosomal translocation. 18, 19 Although FUS-CHOP molecular variants do not appear to influence prognosis in myxoid/round cell liposarcoma, 17 the rarity of EWS-CHOP fusion transcript positive myxoid/round cell liposarcomas has precluded determination of the impact of this variant on clinical outcome. 20
In summary, this case widens the spectrum of sarcomas associated with the der(16)t(1;16) secondary structural change, highlights the advantages of having multiple diagnostic methods available, and illustrates how different genetic approaches may be used to decipher equivocal results.
Acknowledgments
We thank Dr. Gilles Thomas at the Institut Curie in Paris, France for kindly providing the cosmid probes and Patty Cattano for technical assistance.
Address reprint requests to Julia A. Bridge, M.D., Department of Pathology and Microbiology, 983135 Nebraska Medical Center, Omaha, NE 68198-3135. E-mail: jbridge@unmc.edu.
Footnotes
Supported in part by John A. Wiebe Children’s Health Care Fund and NCI-P30 CA36727.
References
- 1.Bridge JA, Roberts CA, Degenhardt J, Walker C, Lackner R, Linder J: Low-level chromosome 12 amplification in a primary lipoma of the lung: evidence for a pathogenetic relationship with common adipose tissue tumors Arch Pathol Lab Med 1998, 122:187-190 [PubMed] [Google Scholar]
- 2.Bridge JA, Fidler ME, Neff JR, Degenhardt J, Wang M, Walker C, Dorfman HD, Baker KS, Seemayer TA: Adamantinoma-like Ewing’s sarcoma: genomic confirmation, phenotypic drift Am J Surg Pathol 1999, 23:159-165 [DOI] [PubMed] [Google Scholar]
- 3.Desmaze C, Zucman J, Delattre O, Melot T, Thomas G, Aurias A: Interphase molecular cytogenetics of Ewing’s sarcoma and peripheral neuroepithelioma t(11;22) with flanking and overlapping cosmid probes Cancer Genet Cytogenet 1994, 74:13-18 [DOI] [PubMed] [Google Scholar]
- 4.Antonescu CR, Tschernyavsky SJ, Decuseara R, Leung DH, Woodruff JM, Brennan MF, Bridge JA, Neff JR, Goldblum JR, Ladanyi M: Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases Clin Cancer Res 2001, 7:3977-3987 [PubMed] [Google Scholar]
- 5.Weiss SW, Goldblum JR: Soft Tissue Tumors 2001:670-687 Mosby, Inc. St. Louis
- 6.Sreekantaiah C, Ladanyi M, Rodriguez E, Chaganti RS: Chromosomal aberrations in soft tissue tumors: relevance to diagnosis, classification, and molecular mechanisms Am J Pathol 1994, 144:1121-1134 [PMC free article] [PubMed] [Google Scholar]
- 7.Tallini G, Akerman M, Dal Cin P, De Wever I, Fletcher CD, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Sciot R, Van den Berghe H, Van den Ven W, Vanni R, Willen H: Combined morphologic and karyotypic study of 28 myxoid liposarcomas: implications for a revised morphologic typing: a report from the CHAMP Group Am J Surg Pathol 1996, 20:1047-1055 [DOI] [PubMed] [Google Scholar]
- 8.Fletcher CD, Akerman M, Dal Cin P, de Wever I, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Sciot R, Tallini G, van den Berghe H, van de Ven W, Vanni R, Willen H: Correlation between clinicopathological features and karyotype in lipomatous tumors: a report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group Am J Pathol 1996, 148:623-630 [PMC free article] [PubMed] [Google Scholar]
- 9.Hattinger CM, Rumpler S, Ambros IM, Strehl S, Lion T, Zoubek A, Gadner H, Ambros PF: Demonstration of the translocation der(16)t(1;16)(q12;q11.2) in interphase nuclei of Ewing tumors Genes Chromosomes Cancer 1996, 17:141-150 [DOI] [PubMed] [Google Scholar]
- 10.Day SJ, Nelson M, Rosenthal H, Vergara GG, Bridge JA: Der(16)t(1;16)(q21;q13) as a secondary structural aberration in yet a third sarcoma, extraskeletal myxoid chondrosarcoma Genes Chromosomes Cancer 1997, 20:425-427 [PubMed] [Google Scholar]
- 11.Mitelman Database of Chromosome Aberrations in Cancer. http://cgap.nci.nih.gov/Chromosomes/Mitelman, 2003
- 12.Tarkkanen M, Karaharju E, Bohling T, Szymanska J, Helio H, Kivioja A, Elomaa I, Knuutila S: Chromosome study of 249 patients examined for a bone tumor Clin Orthop 1993, :117-128 [PubMed] [Google Scholar]
- 13.Stark B, Mor C, Jeison M, Gobuzov R, Cohen IJ, Goshen Y, Stein J, Fisher S, Ash S, Yaniv I, Zaizov R: Additional chromosome 1q aberrations and der(16)t(1;16), correlation to the phenotypic expression and clinical behavior of the Ewing family of tumors J Neurooncol 1997, 31:3-8 [DOI] [PubMed] [Google Scholar]
- 14.Hattinger CM, Rumpler S, Strehl S, Ambros IM, Zoubek A, Potschger U, Gadner H, Ambros PF: Prognostic impact of deletions at 1p36 and numerical aberrations in Ewing tumors Genes Chromosomes Cancer 1999, 24:243-254 [DOI] [PubMed] [Google Scholar]
- 15.Sandberg AA, Bridge JA: Updates on cytogenetics and molecular genetics of bone and soft tissue tumors: Ewing sarcoma and peripheral primitive neuroectodermal tumors Cancer Genet Cytogenet 2000, 123:1-26 [DOI] [PubMed] [Google Scholar]
- 16.Gibas Z, Miettinen M, Limon J, Nedoszytko B, Mrozek K, Roszkiewicz A, Rys J, Niezabitowski A, Debiec-Rychter M: Cytogenetic and immunohistochemical profile of myxoid liposarcoma Am J Clin Pathol 1995, 103:20-26 [DOI] [PubMed] [Google Scholar]
- 17.Antonescu CR, Elahi A, Healey JH, Brennan MF, Lui MY, Lewis J, Jhanwar SC, Woodruff JM, Ladanyi M: Monoclonality of multifocal myxoid liposarcoma: confirmation by analysis of TLS-CHOP or EWS-CHOP rearrangements Clin Cancer Res 2000, 6:2788-2793 [PubMed] [Google Scholar]
- 18.Panagopoulos I, Hoglund M, Mertens F, Mandahl N, Mitelman F, Aman P: Fusion of the EWS and CHOP genes in myxoid liposarcoma Oncogene 1996, 12:489-494 [PubMed] [Google Scholar]
- 19.Panagopoulos I, Lassen C, Isaksson M, Mitelman F, Mandahl N, Aman P: Characteristic sequence motifs at the breakpoints of the hybrid genes FUS/CHOP, EWS/CHOP, and FUS/ERG in myxoid liposarcoma and acute myeloid leukemia Oncogene 1997, 15:1357-1362 [DOI] [PubMed] [Google Scholar]
- 20.Dal Cin P, Sciot R, Panagopoulos I, Aman P, Samson I, Mandahl N, Mitelman F, Van den Berghe H, Fletcher CD: Additional evidence of a variant translocation t(12;22) with EWS/CHOP fusion in myxoid liposarcoma: clinicopathological features J Pathol 1997, 182:437-441 [DOI] [PubMed] [Google Scholar]