Abstract
Adamantinoma of long bones is a rare neoplasm predominantly involving the tibia. Cytogenetic studies of adamantinoma are few. Cytogenetic or molecular cytogenetic analysis of four adamantinomas, and a review of eleven cases in the literature reveals extra copies of chromosomes 7, 8, 12, 19, and 21 as recurrent in this neoplasm. Adamantinoma may be confused with a variety of primary and metastatic epithelial and mesenchymal neoplasms. Observation of these aneuploidies may be useful in establishing the diagnosis of adamantinoma.
Adamantinoma of long bones is a rare low-grade malignant bone tumor most often arising in the tibia, but also occasionally exhibiting synchronous involvement of the ipsilateral fibula. Some adamantinomas have “osteofibrous dysplasia-like” features. 1 Thus, a relationship between adamantinoma and osteofibrous dysplasia (a benign fibro-osseous lesion occurring almost exclusively in the tibia of children less than 10 years of age) has been proposed. This relationship, however, is poorly understood.
Cytogenetic analyses of adamantinoma and osteofibrous dysplasia are few. 2, 3, 4, 5, 6, 7 In this study, the cytogenetic and molecular cytogenetic findings of four cases of adamantinoma and a review of the literature are presented.
Materials and Methods
Case 1
A 37-year-old female presented with a nontender protuberance in the midshaft of the right tibia known since childhood. As a child, she sustained a fracture of the tibia with minimal trauma. The fracture was treated in a cast and healed without complications. For 20 years, the protuberance remained unchanged in size and character and without symptoms. In 1999, however, she sustained a fracture of the tibia once again and with minimal trauma. Plain radiographs demonstrated a pathological fracture through a destructive process including the lesion and protuberance. A biopsy revealed adamantinoma. A radical en bloc resection of the right tibial diaphysis and reconstruction using fresh frozen allograft tibia, plates, and autologous bone graft were performed.
Histopathologically, both the biopsy and resected specimens were composed of epithelial-like cells arranged in large islands or nests embedded in a densely fibrous stroma. Mitotic figures could not be identified. Immunohistochemical staining revealed that the epithelial cells were immunoreactive for cytokeratin (AE1:AE3). The histopathological diagnosis was classic adamantinoma.
Case 2
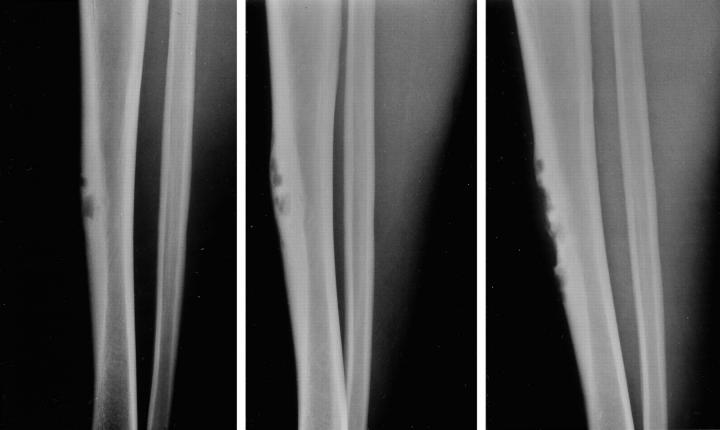
A 20-year-old male sought medical attention because of pain in the anterior aspect of his right leg with recreational running. Four years earlier, the patient had noted a bulge on the anterior border of his right tibia. Plain radiographs revealed a bubbly lesion involving the anterolateral cortex of the midshaft of the right tibia. Comparison with previous radiographs over the past 4 years showed indolent progression (Figure 1) . Magnetic resonance imaging (MRI) demonstrated a multilobulated mass that uniformly enhanced with contrast. A bone scan showed intense uptake confined to the site of the lesion seen in the plain radiographs and revealed no other areas of significant skeletal uptake. An incisional biopsy was followed by a wide local excision and reconstruction.
Figure 1.
The Case 2 lesion involving the anterior cortex has multiple radiolucent defects surrounded by areas of cortical thickening and a thick rim of reactive bone at the base. One can observe indolent progression of the radiolucent areas from the first film taken July, 1995 (left) to subsequent ones obtained May, 1996 (center) and December, 1999 (right).
Microscopically, the lesional tissue was intracortical. At the periphery, the cortex demonstrated enlarged Harversian canals filled with a paucicellular fibroblastic reactive tissue. There was a transition from mature to less mature bone tissue, or so-called zoning. The center of the lesion consisted of a loosely textured fibroblastic proliferation with occasional rounded or oval deposits of nonlamellar bone without noticeable osteoblastic rimming and nests of epithelial-like cells (Figure 2) . Immunohistochemical staining for cytokeratin (AE1:AE3) was positive (Figure 2) . The histopathological diagnosis was differentiated adamantinoma.
Figure 2.
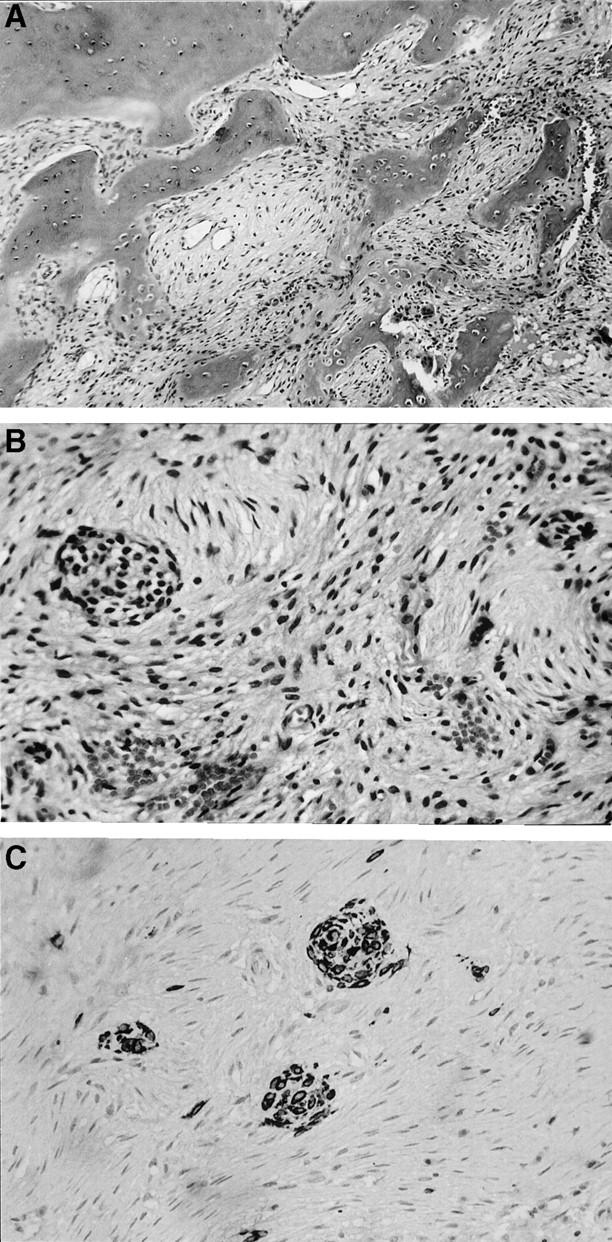
A: The medullary side of a portion of the reactive cortex is at the top of the left hand corner. It is fairly mature, but lacks well-developed Harversian systems and stress lines. Connected to it and protruding toward the underlying lesion, are thickened trabeculae of irregular, immature bone variably rimmed by osteoblasts. Osteoclastic remodeling can be seen in the lower right hand corner. B: The center of the lesion is characterized by a bland proliferation of cells having spindle shaped, often slender, nuclei arranged in a loose storiform pattern. Embedded within this stroma are two nests of epithelial cells. C: An immunohistochemical preparation for cytokeratins highlights three nests of epithelial cells. In addition, multiple keratin reactive single cells within the stroma, not otherwise detectable, can be appreciated.
Case 3
A 16-year-old female was medically evaluated for an enlarging, painful lesion in the midshaft of the right tibia. The MRI demonstrated a lesion occupying the marrow of the diaphysis of the tibial shaft containing multiple fluid-fluid levels. Extensive endosteal erosion, cortical thinning, and a small associated extraosseous soft tissue mass were noted. The ipsilateral fibula was unremarkable. An open biopsy was performed resulting in a tissue diagnosis of adamantinoma. The patient underwent a wide local excision of the midshaft tibia using the contralateral revascularized osteocutaneous fibula as a graft for reconstruction. The resected specimen showed two separate hemorrhagic and partly cystic lesions in the mid and distal tibial shaft, measuring 7.0 and 2.4 cm, respectively, with cortical breakthrough into the adjacent soft tissues. Microscopically both foci had similar features, large nests of epithelioid cells in a basaloid pattern, characteristic of classic adamantinoma. The tumor cells were strongly immunoreactive for 34BE12 and BCL2 and focally immunoreactive for cytokeratin (AE1:AE3).
Case 4
A 14-year-old male was evaluated for increasing pain and swelling of his right leg. His past medical history revealed a long record of lesions involving his right tibia and fibula since the age of 2, including three surgical procedures performed on these lesions at ages 4, 7, and 11. A curettage without graft of the tibia was performed for a pathological fracture at age 4, diagnosed at an outside hospital as a unicameral bone cyst. At age 8, the patient underwent a resection of a fibular lesion, which was diagnosed as osteofibrous dysplasia. At age 11, he had a curettage and grafting of a tibial lesion, which also demonstrated features consistent with osteofibrous dysplasia. Plain films revealed a lytic lesion in the distal tibia, with significant endosteal scalloping. The entire posterior lateral cortex was destroyed, and on MRI a large soft tissue component projecting into the posterior compartment was noted.
Lesional tissue obtained from an initial curettage of the right tibia was diagnostic of classic adamantinoma. The tumor cells were positive for cytokeratins (CAM 5.2, AE1:AE3, and 34BE12). The patient underwent a wide below-knee amputation procedure. The resected specimen showed an 8-cm mass primarily in the soft tissues of the posterior compartment of the right calf, with focal involvement of the posterior cortex of the tibia. The histopathological appearance was similar to that of the curettage specimen. The mitotic count was 20MF/10HPF. No skip lesions or osteofibrous-dysplasia-like changes were identified.
Cytogenetic Analysis
Cytogenetic analyses were performed on representative, sterile tissue removed from the resected specimens of Cases 1 and 2. Standard culture and harvesting procedures were used which have been described previously. 5 Briefly, the tissue was disaggregated mechanically and enzymatically and cultured in RPMI 1640 media supplemented with 20% fetal bovine serum, 1% penicillin/streptomycin-L-glutamine (Irvine Scientific, Santa Ana, CA) for 4 to 7 days. Four hours before harvest, cells were exposed to Colcemid (0.02 mg/ml). After hypotonic treatment (0.074 mol/L KCI for 30 minutes), the preparations were fixed 3 times with methanol and glacial acetic acid (3:1). Metaphase cells were banded with Giemsa trypsin. The karyotypes were expressed according to the International System for Human Cytogenetic Nomenclature 1995. 8
Molecular Cytogenetic Analysis
Fluorescence in situ hybridization (FISH) studies were performed on cytologic touch preparations of Cases 3 and 4 using chromosomes 7-, 8-, 12-, and 19-specific probes (CEP7, Vysis, Inc., Downers Grove, IL; D8Z1 and D12Z1, Oncor, Inc., Gaithersburg, MD; chromosome 19q12–13.1 genomic PAC probe, F1, courtesy of M. Zielenska and J.A. Squire, Toronto 9 ).
Hybridization was performed as previously described. 6 The number of hybridization signals for each specimen was assessed in 200 interphase nuclei with strong and well-delineated signals by two different individuals. A specimen was interpreted as aneuploid for chromosomes 7, 8, 12, and/or 19 if three or more clearly separate signals for each respective probe could be detected in >25% of the cells evaluated (more than two standard deviations above the average false positive rate). To exclude the possibility of triploidy or tetraploidy, the biotinylated probe, D3Z1 (Oncor, Inc.), which binds to the centromeric region of chromosome 3, was used. As an additional control, normal peripheral blood lymphocytes were simultaneously hybridized with chromosomes 3, 7, 8, 12, and 19 probes. Images were prepared using the Applied Image Analysis System (Applied Imaging, Pittsburgh, PA).
Results
Cytogenetics
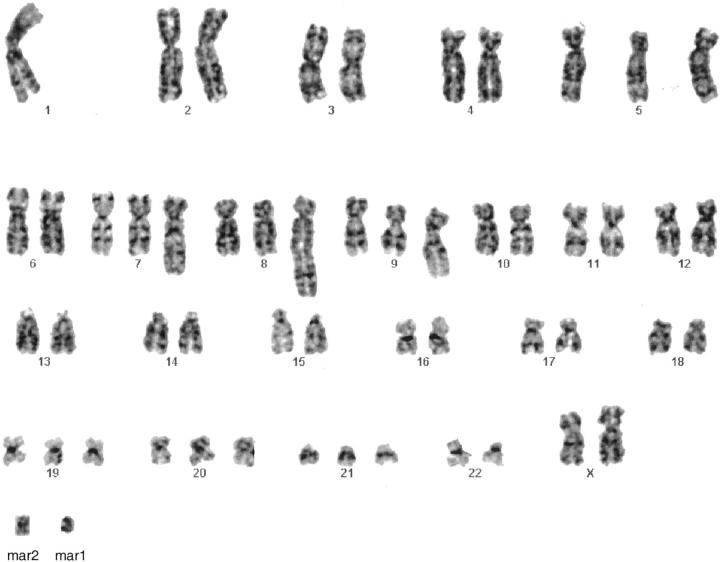
Six metaphase cells were analyzable from Case 1. The following abnormal chromosomal complement was detected in five cells: 54,XX,−1,+5,+der(7)t(?1;7)(?q21;q22), +der(8)t(1;8)(q21;q24.3), +der(9)t(1;9)(p32;q34), +19, +20,+21,+mar1,+mar2 (Figure 3) . One cell was karyotypically normal.
Figure 3.
GTG-banded karyotype of Case 1: 54, XX,−1,+5,+der(7)t(?1;7)(?q21;q22),+der(8)t(1;8)(q21;q24.3),+der(q)t(1:9)(p32;q34),+19,+20,+ 21,+mar1,+mar2.
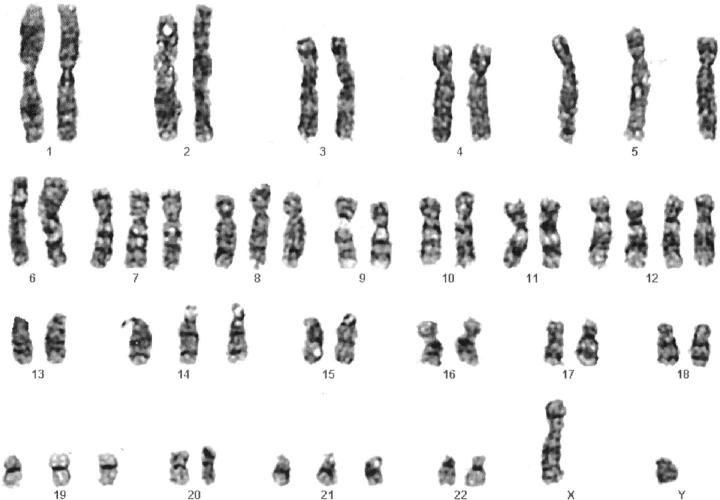
The following primary abnormal clone and subclone were detected in Case 2: 54,XY,+5,+7,+8,+12,+12,+14, +19, +21[6]/53, XY, +4, +5, +7, +8, +12, +14, −16, +17, −18,+21,+mar[2]. A representative karyotype of the primary clone is presented in Figure 4 . Six cells were karyotypically normal.
Figure 4.
GTG-banded karyotype of the primary clone in Case 2: 54, XY,+5,+7,+8,+12,+12,+14,+19,+21.
Molecular Cytogenetics
Trisomy for chromosomes 7, 8, and 19 were detected in 63 to 73% of the cells examined in Case 3. Two copies (diploid copy number) of chromosomes 3 and 12 were detected in 97% and 96% of the cells, respectively, for Case 3.
Assessment of the control probe (chromosome 3) revealed that the majority of cells (83%) in Case 4 were tetraploid. The copy number of chromosome 8 probe signals was five or more in 84% of the 200 cells evaluated and was thus interpreted as abnormal in this case. Four signals (tetraploid copy number) for chromosomes 7, 12, and 19 probes were seen within the control range for this case. Representative interphase cells from both Cases 3 and 4 after FISH analyses are illustrated in Figure 5 .
Figure 5.
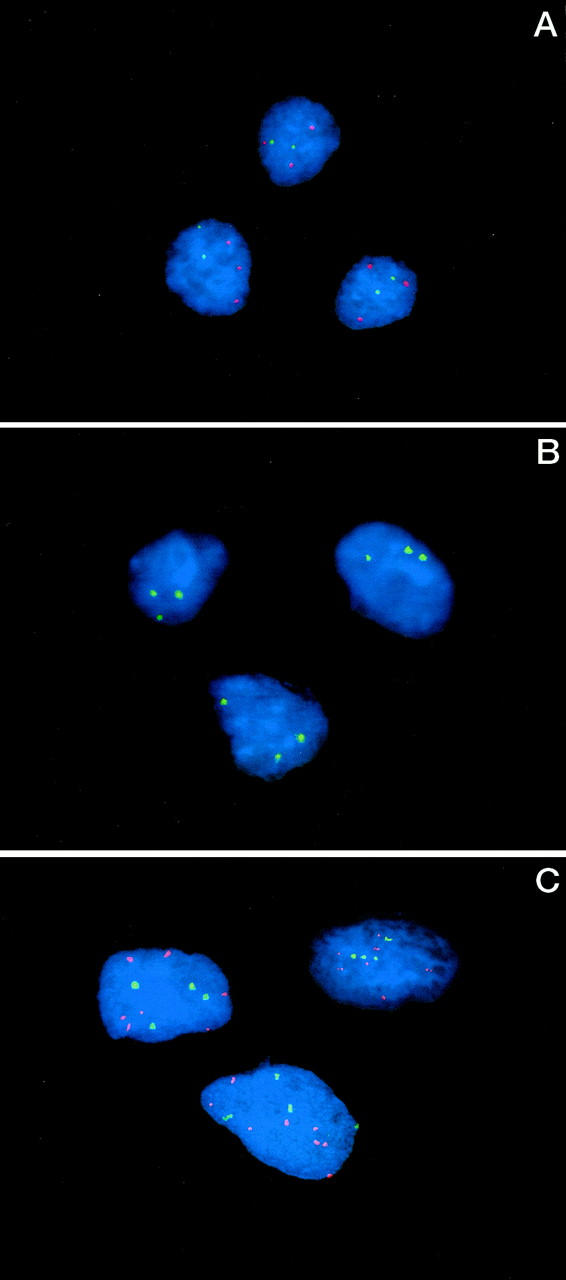
A: FISH analysis performed on cytologic touch preparations of Case 3 revealed three hybridization signals for chromosome 8 (red) and two hybridization signals for chromosome 12 (green). B: FISH analysis performed on cytologic touch preparations of Case 3 revealed three hybridization signals for chromosome 19. C: FISH analysis performed on cytologic touch preparations of Case 4 revealed five to eight hybridization signals for chromosome 8 (red) and four hybridization signals for chromosome 12 (green).
Discussion
Adamantinoma of the appendicular skeleton is a distinct, exceptionally rare neoplasm of controversial histopathogenesis. The first report of an adamantinoma of long bone (“primary myelogenic squamous cell carcinoma of the ulna”) was described by Maier in 1900. 10 This description of a diaphyseal ulnar lesion corresponds to the currently accepted diagnostic criteria for adamantinoma. Only 195 well-documented cases of adamantinoma were identified in a review of the world literature published by Moon and Mori in 1986. 11
Osteofibrous dysplasia of long bone is also a rare lesion, but, in contrast to adamantinoma, it is benign. 12 Certain clinicopathological features, however, such as the radiographic appearance, a proclivity for the tibia, and an association with fibro-osseous tissues are similar in both adamantinoma and osteofibrous dysplasia, supporting the hypothesis of a relationship between these two entities. An adamantinoma subtype has been described (“differentiated adamantinoma”) which occurs intracortically in the first two decades of life and is characterized histologically by a predominantly osteofibrous dysplasia-like pattern and a small inconspicuous component of epithelial elements scattered within the stromal fibroblastic tissue. 13 Classic adamantinoma typically affects patients older than 20 years and exhibits variable histological patterns to include basaloid, spindle, tubular, and squamoid. Czerniak et al 13 proposed the existence of a continuum of fibro-osseous lesions with osteofibrous dysplasia at one end of the spectrum, classic adamantinoma at the other, and differentiated adamantinoma intermediately.
Cytogenetic analysis has contributed greatly to the understanding of the histopathogenesis of many types of neoplasia. Adamantinoma and osteofibrous dysplasia are of unknown histopathogenesis. Unfortunately, because of their rarity, cytogenetic reports concerning these tumors are few. 2, 3, 4, 5, 6, 7 The karyotypic findings of only five cases of classic adamantinoma and two cases of differentiated adamantinoma have been described, 2, 3, 4 Table 1 . An examination of the four current cases and a review of those previously reported reveal that extra copies of chromosomes 7, 8, 12, 19, and/or 21 have been seen in all but one case of classic adamantinoma and one case of differentiated adamantinoma. Extra copies of one or more of these same chromosomes with the exception of chromosome 19 have also been seen in osteofibrous dysplasia (Table 1) , lending further support to an osteofibrous dysplasia/adamantinoma relationship. 4, 5
Table 1.
Cytogenetic Findings in Adamantinoma and Osteofibrous Dysplasia
| Diagnosis | Age, Sex | Karyotype | Reference |
|---|---|---|---|
| OFD | ?, M | 46,XY | Tarkkanen et al. 7 |
| OFD | 11, M | 47,XY,+12 | Bridge et al. 5 |
| OFD | 19, M | 49,XY,+7,+8,+22 | Bridge et al. 5 |
| OFD | 18, F | 52,XX,+5,+7,+7,+8,+21,+21 | Bridge et al. 6 |
| Classic adamantinoma | 15, F | 46,XX,t(1;13;22)(q22;q12;p13), t(15;17)(q12;p13) | Mandahl et al. 2 |
| Classic adamantinoma* | 15, M | 52,XY,t(7;13)(q32;q14),+7,+12,+13,+19,+der(7)t(7;13)(q32;q14),+der(13) t(7;13)(q32;q14) | Sozzi et al 3 |
| Classic adamantinoma† | 22, M | 50,XY,+7,+8,+12,+19 | Hazelbag et al. 4 |
| Classic adamantinoma | 10, M | 50,XY,+7,+8,t(10;12)(p10;p10),+12,+19 | Hazelbag et al. 4 |
| Classic adamantinoma | 37, M | 51,XY,+X,+7,+12,+19,+21/50, XY,+X,+7,+12,+21 | Hazelbag et al. 4 |
| Differentiated adamantinoma | 5, M | 47,XY,+mar/48,XY,+7,+8 | Hazelbag et al. 4 |
| Differentiated adamantinoma | 37, F | 46,XX,t(2;11)(p23;q14)inv(11) (p14q14) | Hazelbag et al. 4 |
| Classic adamantinoma | 37, F | 54,XX,−1,+5,+der(7)t(?1;7) (q21;q22),+der(8)t(1;8) (q21;q24.3),+der(9)t(1;9)(p32;q34),+19,+20,+21,+mar1,+mar2 | Present report |
| Differentiated adamantinoma | 20, M | 54,XY,+5,+7,+8,+12,+12,+14,+19,+21/53,XY,+4,+5,+7,+8,+12,+14,−16,+17,−18,+21,+mar | Present report |
| Classic adamantinoma | 16, F | nuc ish 7cen(D7Z1×3),8cen(D8Z1×3),12cen(D12Z1×2),19q12–13.1(F1×3) | Present report |
| Classic adamantinoma | 14, M | nuc ish 7cen(D7Z1×4),8cen(D8Z1×5–8),12cen(D12Z1×4),19q12–13.1(F1×4) | Present report |
OFD, osteofibrous dysplasia.
The t(7;13) in this case was constitutional.
The following abnormal clone was detected in one additional cell: 49,XY,del(1p),+7,+8,+12(inc).
It will be interesting to see if future studies of osteofibrous dysplasia will also eventually reveal extra copies of chromosome 19, or if this anomaly will remain confined to adamantinoma. Also, it should be noted that structural anomalies to include translocations, deletions, inversions, and marker chromosomes have been detected in both differentiated and classic adamantinomas but not osteofibrous dysplasia. The latter observation suggests that adamantinomas are slightly more karyotypically complex than osteofibrous dysplasia. Often these structural changes are in addition to the common numerical changes (+7, +8, +12, and +21) seen in both adamantinoma and osteofibrous dysplasia. It could be hypothesized that the expansion of the abnormal clone to include structural changes parallels a progression of an osteofibrous dysplasia lesion to an adamantinoma. The observation that adamantinoma may develop from osteofibrous dysplasia has also been supported by others. 1, 5 It was unfortunate that fresh tissue was not submitted for conventional karyotypic analysis on the progressive lesions of the tibia and fibula of Case 4, as it would have been interesting to compare the findings of each lesion.
In summary, these cytogenetic and molecular cytogenetic data reveal that extra copies of chromosomes 7, 8, 12, 19, and 21 are recurrent in adamantinoma. Adamantinoma must be distinguished from a variety of epithelial and soft tissue neoplasms. In particular, epithelial elements of the basaloid, tubular, or squamoid patterns can be misdiagnosed as metastatic carcinoma in a small biopsy specimen. Dominant spindle-cell or small tubular patterns may be confused with a fibrosarcoma or vascular neoplasm. Identification of these recurrent genetic imbalances may ultimately prove to be a useful diagnostic adjunct in adamantinoma.
Acknowledgments
We thank Kimberly Christian for expert secretarial service and Marilu Nelson, Diane Pickering, Patty Cattano, and Michelle Hess for expert technical assistance.
Address reprint requests to Julia A. Bridge, M.D., Department of Pathology and Microbiology, University of Nebraska Medical Center, 983135 Nebraska Medical Center, Omaha, NE 68198-3135. E-mail: jbridge@unmc.edu.
Footnotes
Supported in part by grants from the Orthopaedic Research Education Foundation and the John A. Wiebe, Jr. Children’s Health Care Fund.
References
- 1.Dorfman HD, Czerniak B: Bone Tumors. St. Louis, Mosby, Inc., 1998, pp 949–973
- 2.Mandahl N, Heim S, Rydholm A, Willen H, Mitelman F: Structural chromosome aberrations in an adamantinoma. Cancer Genet Cytogenet 1989, 42:187-190 [DOI] [PubMed] [Google Scholar]
- 3.Sozzi G, Miozzo M, Palma SD, Minelli A, Calderone C, Danesino C, Pastorino U, Pierotti MA, Porta GD: Involvement of the region 13q14 in a patient with adamantinoma of the long bones. Hum Genet 1990, 85:513-515 [DOI] [PubMed] [Google Scholar]
- 4.Hazelbag HM, Wessels JW, Mollevangers P, Van den Berg E, Molenaar WM, Hogendoorn PCW: Cytogenetic analysis of adamantinoma of long bones: further indications for a common histogenesis with osteofibrous dysplasia. Cancer Genet Cytogenet 1997, 97:5-11 [DOI] [PubMed] [Google Scholar]
- 5.Bridge JA, Dembinski A, DeBoer J, Travis J, Neff JR: Clonal chromosomal abnormalities in osteofibrous dysplasia: implications for histopathogenesis and its relationship with adamantinoma. Cancer 1994, 73:1746-1752 [DOI] [PubMed] [Google Scholar]
- 6.Bridge JA, Swarts SJ, Buresh C, Nelson M, Degenhardt JM, Spanier S, Maale G, Meloni A, Lynch JC, Neff JR: Trisomies 8 and 20 characterize a subgroup of benign fibrous lesions arising in both soft tissue and bone. Am J Pathol 1999, 154:729-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarkkanen M, Kaipainen A, Karaharju E, Bohling T, Szymanska J, Helio H, Kivioja A, Elomaa I, Knuutila S: Cytogenetic study of 249 consecutive patients examined for a bone tumor. Cancer Genet Cytogenet 1993, 68:1-21 [DOI] [PubMed] [Google Scholar]
- 8.ISCN: An International System for Human Cytogenetic Nomenclature. Edited by Mitelman F. Basel, Switzerland, Karger, 1995
- 9.Chan AS, Squire JA, Thorner P, Zielenska M: Molecular genetic changes in alveolar soft part sarcoma. Pediatr Pathol Mol Med 2000, 18:529-543 [Google Scholar]
- 10.Maier C: Ein primers myelogenes Platten-epithelkarzinom der Ulna. Beitraege zur klinischen Chirurgie 1900, 26:553-566 [Google Scholar]
- 11.Moon NF, Mori H: Adamantinoma of the appendicular skeleton: updated. Clin Orthop 1986, 204:215-237 [PubMed] [Google Scholar]
- 12.Campanacci M: Osteofibrous dysplasia of long bones: a new clinical entity. Ital J Orthop Traumatol 1976, 2:221-237 [PubMed] [Google Scholar]
- 13.Czerniak B, Rojas-Corona RR, Dorfman HD: Morphologic diversity of long bone adamantinoma: the concept of differentiated (regressing) adamantinoma and relationship to osteofibrous dysplasia. Cancer 1989, 64:2319-2334 [DOI] [PubMed] [Google Scholar]