Abstract
Sepsis continues to be a common source of morbidity and mortality in critically ill patients. Single nucleotide polymorphisms (SNPs) present in genes encoding inflammatory mediators have been associated with predisposition and outcome in this syndrome. The use of high throughput SNP analysis in large epidemiological studies is necessary to more fully understand the genetic underpinnings of this disease. We adapted template-directed dye-terminator incorporation with fluorescence polarization detection (TDI-FP) to the analysis of eight SNPs implicated in mediating the sepsis syndrome: TNF-α (−308), TNF-α (−238), TNF-β (+250), IL-1β (+3953), IL-6 (−174), IL-10 (−592), plasminogen activator inhibitor-1 (PAI-1 (−675)), and TLR4 299 (+1032). Optimization of PCR, amplicon purification, and template-directed dye-terminator incorporation reactions were necessary to achieve acceptable performance characteristics for these assays. Sequence validated samples served as controls. Using this method we were able to assign genotype in 99.3% of assays and identified 64 unique genotypes in samples obtained from 90 individuals. TDI-FP is a flexible and robust method of SNP detection that can be optimized in a systematic fashion. This method has potential advantages compared with other high throughput genotyping techniques and appears well suited to clinical situations requiring analysis of large numbers of samples.
Despite advances in supportive care, the sepsis syndrome—a systemic response to invasive infection and trauma—continues to be a leading cause of resource expenditure, organ failure, and death. 1 Animal and human research suggest that the sepsis syndrome results from excessive inflammation. 2 Numerous clinical trials have examined the therapeutic potential of inhibiting specific inflammatory mediators, such as TNF-α, IL-1, and IL-6, in patients with sepsis. 3, 4, 5 Overall, these anti-cytokine therapies were found to lack efficacy. 3, 4, 5 Therapies that inhibit inflammation may be of greater benefit if more specifically targeted to patient populations at high risk of death. 4, 5, 6, 7, 8 Accordingly, recent investigations have focused on efforts to better understand the mechanisms that control the inflammatory response in sepsis, and to develop refined means of identifying patients predisposed to severe disease.
Like many illnesses, the sepsis syndrome appears to have a genetic component. 9, 10, 11 Single nucleotide polymorphisms (SNPs) present in the promotor region of TNF-α, a pivotal mediator of the sepsis response, have been associated with increased lethality in this syndrome. 12, 13, 14 Likewise, SNPs present in other cytokine genes have been linked to clinical outcome in many infectious and inflammatory conditions. 15, 16, 17, 18, 19, 20 The complexity of the inflammatory response coupled with the large number of clinical factors that may influence outcome in sepsis require that genetic analysis be conducted in a large, diverse population of septic patients to more fully characterize the genetic contribution to this illness. The Genetic Predisposition to Severe Sepsis (GenPSS) study is a National Institutes of General Medical Sciences (NIGMS)-supported multi-institutional, epidemiological study with the goal of performing SNP analysis of functional variants in inflammatory cytokine genes in several thousand patients with sepsis. To date, no genetic epidemiological study of this scope has been undertaken in patients with this disease.
Our first task in conducting the GenPSS study has been to adapt template-directed dye-terminator incorporation with fluorescent polarization-detection (TDI-FP), a high throughput genotyping method, to detection of SNPs present in genes encoding sepsis mediators. 21 In this single base extension technique, an oligonucleotide probe is designed to anneal immediately 5′ to a SNP of interest in PCR-amplified product. In the presence of DNA polymerase and fluorescently labeled dideoxyribonucleoside triphosphates (ddNTPs), the probe is extended by a single base. The specific ddNTP incorporated is dictated by the polymorphic site in the target DNA sequence and results in termination of the extension reaction. Fluorescence polarization (FP), the property that fluorescent molecules emit polarized fluorescent light when excited by plane-polarized light, is used to identify the ddNTP incorporated and assign genotype. 22 Because the FP of a solution reflects the summation of the FP of all individual species present in that solution, optimal genotype discrimination by TDI-FP occurs when conditions have been optimized resulting in the template-directed incorporation reaction being driven to completion. 22
We describe our experience with TDI-FP as well as the steps in PCR amplification, amplicon purification, and TDI necessary to optimize reaction conditions. While this method has been previously described in the context of small pilot studies, the feasibility of adapting this technique for use in large-scale epidemiological investigations has not been previously reported. 22 Our findings suggest that TDI-FP is a robust method of high throughput SNP detection that is potentially adaptable to a large number of clinical settings.
Materials and Methods
Patients
While the goal of the GenPSS is to perform SNP analysis on samples derived from patients with sepsis as defined by standard criteria, 23 we adapted TDI-FP methodology using anonymized archived DNA specimens purified from whole blood by standard salt precipitation technique (Puregene DNA isolation kit, Gentra Systems, Inc., Minneapolis, MN). 24 The GenPSS study has been approved by the Human Studies Committee of Washington University School of Medicine (IRB no. 00–0358).
PCR
We developed assays for eight SNPs present in inflammatory cytokine genes implicated as mediators of the sepsis response. These include two SNPs present in the promotor region of TNF-α (TNF-α (−308) and TNF-α (−238)), SNPs present in the first intron of TNF-β (TNF-β(+250)), exon 5 of IL-1β (IL-1 (+3953)), in the promotor regions of IL-6 (IL-6 (−174)), IL-10 (IL-10 (−592)), and plasminogen activator inhibitor-1 (PAI-1 (−675)), and at codon 299 in toll-like receptor 4 (TLR4299 (+1032). 17, 20, 25, 26, 27, 28 Genomic DNA (5 to 10 ng) was amplified in a mixture containing 2.5 μl TaqPCR Master Mix (Qiagen, Valencia, CA), 0.05 μl 50 mmol/L mixture of forward and reverse primer (Genosys, Sigma, St. Louis, MO), 0.45 μl water, according to the conditions indicated in Table 1 . To optimize the TDI-FP technique, PCR primers and conditions were selected to generate a relatively pure PCR product of 400 bp or smaller. All reactions were performed in 96-well, black-skirted reaction plates (MJ Research, Waltham, MA) using a Tetrad thermal cycler (MJ Research).
Table 1.
PCR Primers, Product Sizes, and Conditions
| Loci | Primers† | PCR product size | PCR conditions‡ |
|---|---|---|---|
| TNF-α (−308) | CTCAGGACTCAACACAGCTT (F) (−490) | 383 bp | 95°C, 5 min; 37 cycles of (95°C, 30 sec, |
| TCTGGAGGAAGCGGTAGTGG (R) (−107) | 60°C, 30 sec, 72°C, 45 sec), 72°C, 5 min | ||
| TNF-α (−238) | GTTCAGCCTCCAGGGTCCTACACA (F) (−300) | 131 bp | 95°C, 5 min; 35 cycles of (95°C, 30 sec, |
| GGGATTTGGAAAGTTGGGGACACA (R) (−171) | 69°C, 30 sec, 72°C, 45 sec), 72°C, 5 min | ||
| TNF-β (+250) | TTCCTTCTCTGTCTCTGACT (F) (+178) | 168 bp | 95°C, 5 min; 37 cycles of (95°C, 30 sec, |
| AGAGAGATCGACAGAGAAGG (R) (+345) | 60°C, 30 sec, 72°C, 45 sec), 72°C, 5 min | ||
| IL-1β (+3953) | GTTGTCATCAGACTTTGACC (F) (+3888) | 131 bp | 95°C, 5 min; 37 cycles of (95°C, 30 sec, |
| TTCAGTTCATATGGACCAGA (R) (+4018) | 60°C, 30 sec, 72°C, 45 sec), 72°C, 5 min | ||
| IL-6 (−174) | TTGTCAAGACATGCCAAAGTG (F) (−305) | 244 bp | 95°C, 5 min; 37 cycles of (95°C, 30 sec, |
| TCAGACATCTCCAGTCCTATA (R) (−7) | 60°C, 30 sec, 72°C, 45 sec), 72°C, 5 min | ||
| IL-10 (−592) | GGACAGCTGAAGAGGTGGAA (F) (−680) | 121 bp | 95°C × 5 min; 37 cycles of (95°C, 30 sec, |
| GGCAGTCACCTTAGGTCTCT (R) (−469) | 60°C, 30 sec, 72°C, 45 sec), 72°C, 5 min | ||
| PAI-1 (−675)* | GGTTGTTGACACAGAGAGCC (F) (−734) | 168 bp | 95°C, 5 min; 37 cycles of (95°C, 30 sec, |
| GCCACGTGATTGTCTAGGTT (R) (−565) | 60°C, 30 sec, 72°C, 45 sec), 72°C × 5 min | ||
| TLR4299 (+1032) | GTATTCAAGCTCTGGCTGGT (F) (+901) | 224 bp | 95°C, 5 min; 36 cycles of (95°C, 30 sec, |
| CAATAGTCACACTCACCAGG (R) (+1125) | 56°C, 30 sec, 72°C, 65 sec), 72°C, 5 min |
Insertion deletion polymorphism.
Primers are listed in a 5′-3′ orientation (F, forward primer; R, reverse primer). The numbers in parentheses refer to the position of the 5′ terminus relative to the SNP of interest.
Following amplification, PCR products were stored at 4°C.
Amplicon Purification
To eliminate single-stranded oligonucleotides and unincorporated dNTPs which may lessen the specificity of the TDI-FP reaction, E. coli exonuclease I (USB, Cleveland, OH) (0.1 μl), shrimp alkaline phosphatase (Roche Molecular Biochemicals, Indianapolis, IN) (1.0 μl), 10X SAP reaction buffer (0.2 μl), and water (0.7 μl) were added to the total volume of PCR product (5 μl) and incubated at 37°C for 40 minutes, followed by 80°C for 15 minutes for enzyme inactivation.
Template-Directed Dye-Terminator Incorporation Reaction
For TNF-α (−308), TNF-α (−238), TNF-β (+250), IL-1 (+3953), IL-10 (−592), PAI-1 (−675), and TLR4 299 (+1032), the digested PCR product was combined with 0.05 μl acyclopol, 2.0 μl 10X reaction buffer, 1.0 μl of the specific dye terminator combination (Perkin Elmer Life Sciences, Inc, Boston, MA), 0.5 μl 10 μmol/L oligonucleotide probe (Genosys, Sigma, The Woodlands, TX), and 9.45 μl water. For IL-6 (−174), the reaction mixture contained 0.025 μl acyclopol, 2.0 μl 10X reaction buffer, 0.5 μl of the specific dye terminator combination, 0.25 μl 10 mmol/L oligonucleotide probe, and 4.225 μl water. For all assays, the final reaction volume was 20 μl. The reaction mixtures were then incubated according to the conditions specified in Table 2 . Annealing temperatures were selected to be approximately 10°C below the Tm of the oligonucleotide probe. 29
Table 2.
TDI-FP Oligonucleotide Probes, Dye-Terminator Combinations, and Reaction Conditions
| Loci | Base change | TDI-FP probes† | Dye terminator | TDI-FP reaction conditions‡ |
|---|---|---|---|---|
| TNF-α (−308) | G → A | GAGGCAATAGGTTTTGAGGGGCATG (−333) (F) | G/A | 95°C, 120 sec, 30 cycles of (94°C, 15 sec, 61°C, 30 sec) |
| TNF-α (−238) | G → A | GGCCCAGAAGACCCCCCTCGGAATC (−263) (F) | G/A | 95°C, 120 sec, 30 cycles of (94°C, 15 sec, 69°C, 30 sec) |
| TNF-β (+250) | A → G | TGTCACACATTCTCTGTTTCTGCCATG (+250) (F) | G/A | 95°C, 120 sec, 30 cycles of (94°C, 15 sec, 61°C, 30 sec) |
| IL-1β (+3953) | C → T | TGCTCCACATTTCAGAACCTATCTTCTT (+3925) (F) | C/T | 95°C, 120 sec, 30 cycles of (94°C, 15 sec, 58°C, 30 sec) |
| IL-6 (−174) | G → C | TTTTCCCCCTAGTTGTGTCTTGC (−197) (F) | G/C | 95°C, 120 sec, 30 cycles of (94°C, 15 sec, 58°C, 30 sec) |
| IL-10 (−592) | C → A | TTTCCAGAGACTGGCTTCCTACAG (−593) (R) | G/T | 95°C, 120 sec, 30 cycles of (94°C, 15 sec, 52°C, 30 sec) |
| PAI-1 (−675)* | 4G/5G* | CAGAGACACTCTGGACACGTGGGG (−703) (F) | G/A | 95°C, 120 sec, 30 cycles of (94°C, 15 sec, 63°C, 30 sec) |
| TLR4299 (+1032) | A → G | GATTAGCATACTTAGACTACTACCTCGATG (+1032) (F) | G/A | 95°C, 120 sec, 30 cycles of (94°C, 15 sec, 53°C, 30 sec) |
Insertion deletion polymorphism.
Probes are listed in 5′-3′ orientation (F, forward primer; R, reverse primer). The numbers refer to the position of the 5′ terminus relative to the SNP of interest.
All reaction products are stored at 4°C.
Fluorescence Polarization Determination
Fluorescent polarization (FP) values were directly measured using an Analyst fluorescence reader (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths of 580 nm and 605 nm for R110, and 552 nm and 575 nm for TAMARA, respectively. For IL-6 (−174), 0.1 μl single-stranded DNA binding protein (USB, Cleveland, OH), 0.2 μl 10X SAP reaction buffer, and 0.7 μl water was added to the TDI reaction product and incubated at 37°C for 60 minutes before FP determination. For the remainder of the SNPs analyzed, FP was determined immediately following the TDI reaction.
Between reaction steps (eg, PCR, amplicon purification, TDI amplification, and incubation with single-stranded DNA binding protein), reaction plates were thermally sealed (Abgene Thermal Sealer, Surrey, UK) to minimize evaporative loss and cross contamination, and pulse centrifuged.
Allele Assignment and Statistical Analysis
Allele assignment was made using standard software (Perkin Elmer Life Sciences, Inc at http://lifesciences.perkinelmer.com/products/snp.asp). Statistical analysis of FP values was performed using standard techniques (analysis of variance, t-test as appropriate) and software (GraphPad Prism, San Diego, CA). Samples that were indeterminate following the first analysis were re-analyzed using identical methodology. Samples were considered of indeterminate genotype if allele assignment could not be made after two repetitions of TDI-FP methodology. To confirm the accuracy of our approach, sequence-verified controls for each genotype were included in each TDI-FP assay.
Results
Graphical Analysis
Plots of TDI-FP assays for all loci tested are presented in Figure 1 . The scatter plot for TNF-α (−308) (Figure 1A) demonstrates a typical pattern with the data segregated into four distinct groups. Negative controls, which lack DNA, have low FP values (measured as millipolarization (mP), a dimensionless unit) for both fluorophores analyzed (eg, R110 and TAMARA) and are situated near the origin of the plot (in the left graph lower quadrant). Samples homozygous for the G allele have elevated FP values for R110, consistent with incorporation of this fluorophore, but low values for TAMARA. These samples cluster in the right lower graph quadrant. Conversely, samples that are homozygous for the A allele have elevated FP values for TAMARA, consistent with its incorporation, but low FP values for R110. These samples cluster in the left upper graph quadrant. For heterozygous samples, both R110 and TAMARA are incorporated, resulting in clustering of samples in the right upper graph quadrant. Samples that plotted outside these clusters were considered of indeterminate genotype.
Figure 1.
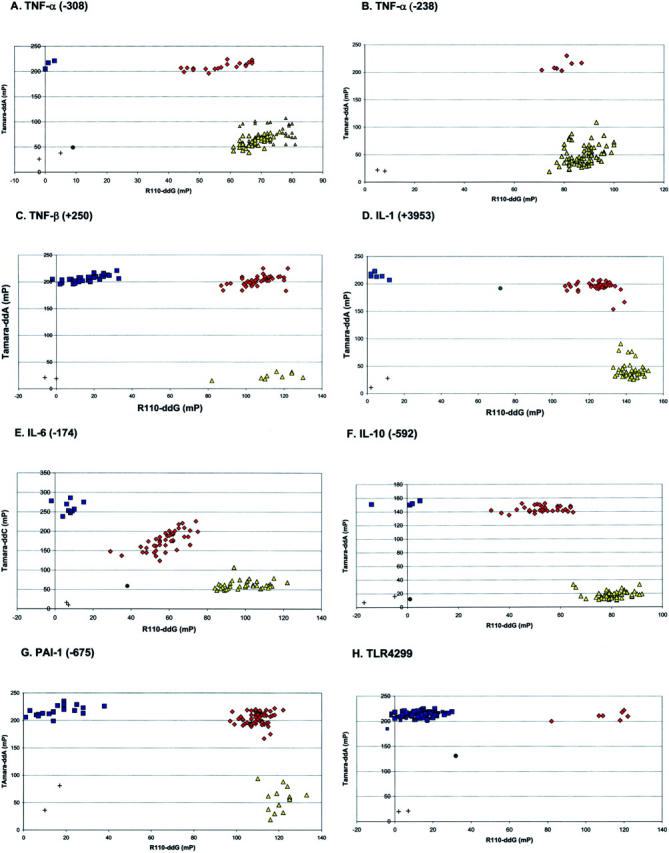
Scatter plots for each SNP are shown in panels A-H. Blue squares represent homozygotes detected by TAMARA-dye, yellow triangles represent homozygotes detected by R110 dye, red diamonds represent heterozygotes detected by incorporation of both TAMARA and R110, green circles represent indeterminate samples, and “+ ” represents negative controls. (Refer to Results for details.
FP Analysis
FP values for R110 and TAMARA are presented in Table 3 . While the FP values measured for R110 differed significantly comparing genotypes (P < 0.0001 for all, analysis of variance), the magnitude of the difference in FP values comparing W/W and W/w genotypes for TNF-α (−238) is small. These genotypes are easily distinguished on the basis of TAMARA uptake. Likewise, for TAMARA, with the exception of TLR4 299 (+1032), FP values differed significantly comparing genotypes (P < 0.0001 for all, analysis of variance). The FP values for TLR4 299 (+1032) comparing wild-type and heterozygote individuals, did not differ significantly (P = 0.25). However, these genotypes were easily discriminated on the basis of R110 incorporation (Figure 1H) .
Table 3A.
Mean (± SEM) FP Values for R110 Incorporation for Each Genotype
| Loci | Genotype* | P-value† | ||
|---|---|---|---|---|
| W/W | W/w | w/w | ||
| TNF-α (−308) | 70.7 (± 0.7) | 57.0 (± 1.7) | 1.3 (± 0.8) | P < 0.0001 |
| TNF-α (−238)‡ | 87.9 (± 0.6) | 79.1 (± 2.0) | N/A | P < 0.0001 |
| TNF-β (± 250) | 15.5 (± 1.4) | 105.8 (± 1.3) | 117.0 (± 2.6) | P < 0.0001 |
| IL-1 (+3953) | 142.3 (± 0.7) | 122.9 (± 1.7) | 5.5 (± 1.6) | P < 0.0001 |
| IL-6 (−174) | 100.0 (± 1.7) | 58.0 (± 1.4) | 7.3 (± 1.4) | P < 0.0001 |
| IL-10 (−592) | 80.9 (± 0.8) | 52.3 (± 1.4) | 1.5 (± 4.2) | P < 0.0001 |
| PAI-1 (−675) | 15.5 (± 2.1) | 109.8 (± 0.7) | 120.6 (± 1.5) | P < 0.0001 |
| TLR4299 | 13.2 (± 0.8) | 111.0 (± 5.3) | N/A | P < 0.0001 |
W denotes the more frequent genotype, w denotes the variant genotype (SNP).
P-value (ANOVA) comparing genotypes.
While these results are statistically different, W/W and W/w genotypes are more readily distinguished on the basis of differences in TAMARA incoporation.
Table 3B.
Mean (± SEM) FP Values for TAMARA Incorporation for Each Genotype
| Loci | Genotype* | P-value† | ||
|---|---|---|---|---|
| W/W | W/w | w/w | ||
| TNF-α (−308) | 68.6 (± 1.9) | 210.0 (± 1.6) | 214.3 (± 4.8) | P < 0.0001 |
| TNF-α (−238) | 48.8 (± 1.8) | 212.1 (± 3.6) | N/A | P < 0.0001 |
| TNF-β (+250) | 206.2 (± 0.97) | 201.9 (± 1.4) | 25.7 (± 2.0) | P < 0.0001 |
| IL-1 (+3953) | 42.7 (± 2.2) | 195.0 (± 1.4) | 214.8 (± 2.2) | P < 0.0001 |
| IL-6 (−174) | 61.6 (± 1.8) | 175.9 (± 3.7) | 260.5 (± 5.0) | P < 0.0001 |
| IL-10 (−592) | 18.2 (± 0.7) | 143.7 (± 1.4) | 152.3 (± 1.3) | P < 0.0001 |
| PAI-1 (−675) | 217.6 (± 1.8) | 204.1 (± 1.5) | 56.9 (± 5.9) | P < 0.0001 |
| TLR4 299‡ | 213.6 (± 0.7) | 210.6 (± 3.0) | N/A | 0.2511 |
W denotes the more frequent genotype, w denotes the variant genotype (SNP).
P-value (ANOVA) comparing genotypes.
No significant difference comparing W/W and W/w genotypes for TLR4 299 is detected. These genotypes are readily distinguished on the basis of R110 incorporation.
Indeterminate Genotypes
The proportion of samples that were of indeterminate genotype following initial assay for each SNP are as follows: TNF-α(−308)−13/90 (14.4%), TNF-α(−238)−7/90 (7.8%), TNF-β (+250)−6/90 (6.7%), IL-1 (+3953)−11/90 (12.2%), IL-6(−174)−10/90 (11.1%), IL-10 (−592)−16/90 (17.7%), PAI-1 (−675)−9/90 (10.0%), and TLR4299 (+1032)−2/90 (2.2%). After these indeterminate samples were re-assayed using identical technique, we were able to assign genotype in 99.3% of the samples and determine 64 unique genotypes. Allele frequencies are presented in Table 4 .
Table 4.
Genotype Frequencies*
| Loci | W/W | W/w | w/w |
|---|---|---|---|
| TNF-α (−308) | 3/90 (3.3%) | 21/90 (23.3%) | 66/90 (73.3%) |
| TNF-α (−238) | 83/90 (92.2%) | 7/90 (7.8%) | 0/90 (0.0%)† |
| TNF-β (+250) | 37/90 (41.1%) | 44/90 (48.9%) | 9/90 (10%) |
| IL-1 (−3953) | 5/90 (5.5%) | 42/90 (46.7%) | 43/90 (47.8%) |
| IL-6 (−174) | 37/90 (41.1%) | 43/90 (47.8%) | 10/90 (11.1%) |
| IL-10 (−592) | 4/90 (4.4%) | 35/90 (38.9%) | 51/90 (56.7%) |
| PAI-1 (−675) | 20/90 (22.2%) | 57/90 (63.3%) | 14/90 (15.5%) |
| TLR4299 | 83/90 (92.2%) | 7/90 (7.8%) | 0/90 (0%)† |
W denotes the more frequent genotype, w denotes the variant genotype (SNP).
Given their infrequent nature, w/w genotypes for TNF-α (−238) and TLR4 299 were not observed.
Discussion
We selected TDI-FP for our study because we sought a high throughput method of SNP detection that is flexible, simple to optimize, and robust. Using this technique, we found that we could unambiguously assign genotype in 99.3% of samples. To achieve these results, several aspects of this technique, including PCR amplification, amplicon purification, and the template-directed dye-terminating incorporation reaction, required manipulation.
To minimize non-specific annealing between TDI oligonucleotide probes and PCR amplification products, we selected PCR primers that amplified small DNA segments (121 to 383 bp in this study), and optimized PCR conditions so as to generate homogeneous PCR targets. The influence of the size of the PCR product is illustrated by our assay for TNF-α(−238). Though this polymorphic site is contained within the genomic segment amplified by the primers used in our assay for TNF-α(−308), it was necessary for us to select PCR primers that amplified a smaller segment containing this SNP for us to obtain acceptable results. We typically amplified 10 ng of genomic DNA. However, for many assays, excellent discrimination between genotypes was obtained over a broad range of DNA concentrations (data not shown). In contrast, for one SNP we evaluated, IL-6 (−174), the TDI assay appeared more sensitive to the amount of genomic DNA present and would only provide acceptable results if smaller amounts of DNA were used. Following PCR amplification, reaction products were digested with a mixture of shrimp alkaline phosphatase and exonuclease 1 to eliminate single-stranded oligonucleotides and unincorporated dNTPs.
We selected TDI oligonucleotide probes that were at least 24 nucleotides in length for our assays. This length theoretically corresponds to a molecular weight that provides optimal discrimination between incorporated and unincorporated ddNTPs by FP analysis, and favors specificity in annealing adjacent to the SNP of interest. 22 Our general approach was to select forward and reverse probes, 24 and 30 nucleotides in length, and to determine which of these nucleotides provided the greatest discrimination in our system. It has not been necessary in our experience to use TDI probes greater than 30 nucleotides in length, though for selected SNPs, there may be some advantages to using these longer probes to enhance the specificity of annealing between the oligonucleotide and PCR product. We set the annealing temperature of the TDI reaction to be 10°C lower than the melting temperature of the TDI probe, though other investigators have described acceptable results using less stringent reaction conditions. 22, 29 Finally, the total FP measured for a solution is a summation of the FP for the individual species in solution. The FP contributed by the incorporated and unincorporated ddNTPs cannot be distinguished. Allele discrimination by TDI-FP will be optimal when 100% incorporation of the ddNTPs has occurred. Methods of assuring that the TDI reaction is driven to completion include altering the number of TDI reaction cycles and adjusting the concentrations of constituents in solution. In our current study, we found that 30 reaction cycles were adequate for all assays and that increasing the number of reaction cycles for some assays resulted in less ability to discriminate among genotypes, presumably due to mis-incorporation of ddNTPs. We found that a lower concentration of constituents was necessary to optimize the IL-6 (−174) reaction. Finally, the addition of single-stranded DNA binding protein results in an increase in the molecular weight of single-stranded DNA, thus allowing for greater discrimination between TDI oligonucleotide probes and unincorporated ddNTPs. 22 The results of most of our assays were acceptable without the addition of this reagent.
While we were ultimately able to assign genotype in 99.3% of samples, one limitation of this technique is that as many as 14% of samples yielded an indeterminate genotype on the initial assay and required re-analysis. The first pass failure rate of this technique has not been previously reported, but is higher than that reported by Hsu et al 30 for a high throughput technique based on the Invader assay. Whether the first pass characteristics of TDI-FP can be improved with further refinement of assay conditions or experience with the technique requires further study. Further, a limitation of our study was that we confirmed genotype by including sequence-confirmed controls in each assay, but did not confirm the TDI-FP results for each sample assayed. Others have reported 100% concordance comparing TDI-FP with other methodologies. 22, 30 For this reason, we consider the accuracy of TDI-FP methodology established.
Studies reported to date examining the contribution of genetic variability to predisposition and outcome in sepsis have been limited both in the numbers of patients enrolled and in the number of genetic loci examined. In contrast, a large number of SNPs have now been reported in genes implicated in the inflammatory response. Though we assayed for only eight SNPs, we identified 64 unique genotypes among 90 samples. This degree of genetic heterogeneity emphasizes the need for clinical studies that possess sufficient statistical power so that the relationship between common genotypes and clinical outcome can be accurately defined.
In summary, a number of high throughput approaches to SNP detection have been described. No single approach, however, has been confirmed in a setting requiring analysis of a large volume of clinical samples. 31 We believe that the TDI-FP methodology has several advantages over other techniques. The TDI-FP assay does not require the use of specially modified probes or reagents, the PCR and incorporation reaction conditions can be readily optimized, and FP detection occurs directly in solution, and does not require separation of free from bound components. Using this technique, we were able to assign genotype in over 99% of the samples tested. The long-term goal of our study is to better understand the biological significance of functional polymorphisms present in genes encoding inflammatory mediators in patients with the sepsis syndrome. However, this methodology has many potential applications, including genetic analysis of other illnesses in which abnormalities of the inflammatory cascade are implicated, or more generally, to settings requiring a flexible, high throughput method of SNP detection.
Address reprint requests to Bradley D. Freeman, M.D., Department of Surgery, Washington University School of Medicine, Box 8109, St. Louis, MO 63110. E-mail: freemanb@msnotes.wustl.edu.
Footnotes
Supported in part by GM00691 (to B.D.F.) and GM61696 (to T.G.B. and B.A.Z.)
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001, 29:1303-1310 [DOI] [PubMed] [Google Scholar]
- 2.Natanson C, Hoffman WD, Suffredini AF, Eichacker PQ, Danner RL: Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med 1994, 120:771-783 [DOI] [PubMed] [Google Scholar]
- 3.Freeman BD, Eichacker PQ, Natanson C: The role of inflammation in sepsis and septic shock: a meta-analysis of both clinical and pre-clinical trials of anti-inflammatory therapies. Gallin J Snyderman R eds. Inflammation: Basic Principles and Clinical Correlates ed 3 1999:965-976 Lippincott Williams & Wilkins Philadelphia
- 4.Freeman B, Natanson C: Anti-inflammatory therapies in sepsis and septic shock. Expert Opinion on Investigational Drugs 2000, 9:1651-1663 [DOI] [PubMed] [Google Scholar]
- 5.Freeman BD, Parrillo JE, Natanson C: Septic shock and multiple organ failure. Parrillo JE Dellinger P eds. Critical Care Medicine ed 2 2001. Mosby Publishing Co St. Louis, MO
- 6.Kay CA: Can better measures of cytokine responses be obtained to guide cytokine inhibition. Cambridge Health Institutes’ Designing Better Drugs and Clinical Trials for Sepsis/SIRS: Reducing Mortality to Patients and Suppliers. Washington, DC, 1996
- 7.Freeman BD, Buchman TG: Coagulation inhibitors in the treatment of sepsis. Expert Opinion on Investigational Drugs 2002, 11:69-74 [DOI] [PubMed] [Google Scholar]
- 8.Zeni F, Freeman BD, Natanson C: Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 1997, 25:1095-1100 [DOI] [PubMed] [Google Scholar]
- 9.Sorensen TIA, Nielsen GG, Anderson PK, Teasdale TW: Genetic and environmental influences on premature death in adult adoptees. N Engl J Med 1988, 318:727-732 [DOI] [PubMed] [Google Scholar]
- 10.Tabrizi RT, Zehnbauer BA, Buchman TG, Freeman BD: Genetic markers in sepsis. J Am Coll Surg 2001, 192:106-117 [DOI] [PubMed] [Google Scholar]
- 11.Freeman BD, Zehnbauer B: Genetic susceptibility to infection and sepsis. Eichacker PQ Pugin J eds. Perspectives on Critical Care Infectious Disease 2001:69-80 Kluwer Academic Publishers Boston
- 12.Stuber F, Peterson M, Bokelmann F, Schade U: A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor concentrations and outcome of patients with severe sepsis. Crit Care Med 1996, 24:381-384 [DOI] [PubMed] [Google Scholar]
- 13.Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, Cheval C, Monchi M, Teboul JL, Riche F, Leleu G, Arbibe L, Mignon A, Delpech M, Dhainaut JP: Association of TNF2, a TNF promotor polymorphism, with septic shock susceptibility and mortality: a multi-center study. J Am Med Assoc 1999, 282:561-568 [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Short J, Parrillo JE: Genetic factors in septic shock. J Am Med Assoc 1999, 282:579-581 [DOI] [PubMed] [Google Scholar]
- 15.Cabrera M, Shaw MA, Sharples C, Williams H, Castes M, Convit J, Blackwell JM: Polymoprhism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med 1995, 182:1259-1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fishman D, Faulds G, Jeffrey R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P: The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 1998, 102:1369-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermans PWM, Hibberd ML, Booy R, Daramola O, Hazelzet JA, de Groot R, Levin M: 4G/5G promotor polymorphism in the plasminogen-activator inhibitor-1 gene and outcome of meningococcal disease. Lancet 1999, 354:556-600 [DOI] [PubMed] [Google Scholar]
- 18.Majetschak M, Flohe S, Obertacke U, Schroder J, Staubach K, Nast-Kolb D, Schade FU, Stuber F: Relation of a TNF gene polymorphism to severe sepsis in trauma patients. Ann Surg 1999, 230:207-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pociot F, Briant L, Jongeneel CV: Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF-α and TNF-β by human mononuclear cells: a possible link to insulin-dependent diabetes mellitus. Eur J Immunol 1993, 23:224-231 [DOI] [PubMed] [Google Scholar]
- 20.Westendorp RGJ, Hottenga JJ, Slagboom PE: Variation in plasminogen-activator-inhibitor-1 gene and risk of meningococcal septic shock. Lancet 1999, 354:561-563 [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Levine L, Kwok P-Y: Fluorescence polarization in homogeneous nucleic acid analysis. Genome Res 1999, 9:492-498 [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu TM, Chen X, Duan S, Miller RM, Kwok PY: Universal SNP genotyping assay with fluorescence polarization detection. BioTechniques 2001, 31:560-570 [DOI] [PubMed] [Google Scholar]
- 23.: Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee: American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992, 20:864-874 [PubMed] [Google Scholar]
- 24.Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R: Efficacy and safety of monoclonal antibody to human tumor necrosis factor-α in patients with sepsis syndrome. J Am Med Assoc 1995, 273:934-941 [PubMed] [Google Scholar]
- 25.Morse RH, Olomolaiue OO, Wood NAP, Keen LJ, Bidwell JL: Induced heteroduplex genotyping of TNF-a, IL-1b, IL-6, and IL-10 polymorphisms associated with transcriptional regulation. Cytokine 1999, 11:789-795 [DOI] [PubMed] [Google Scholar]
- 26.Tang G, Huang S, Yien H, Chen C, Wu C, Chi C, Wu C, Lui W, Chiu J, Lee T: Tumor necrosis factor gene polymorphism and septic shock in surgical infection. Crit Care Med 2000, 28:2733-2736 [DOI] [PubMed] [Google Scholar]
- 27.Zheng C, Huang DR, Bergenbrant S, Sunblad A, Osterborg A, Bjorkholm M, Holm G, Yi Q: Interleukin 6, tumor necrosis factor α, interleukin 1 β, and interleukin 1 receptor antagonist promotor or coding gene polymorphisms in multiple myeloma. Br J Haematol 2000, 109:39-45 [DOI] [PubMed] [Google Scholar]
- 28.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JK, Jones M, Frees K, Watt JL, Schwartz DA: TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 2000, 25:187-191 [DOI] [PubMed] [Google Scholar]
- 29.Department of Psychiatry Genotyping Shared Facility: SNP Genotyping. 2002, http://psy-svr1.bsd.uchicago.edu/geno/snp/snp.html
- 30.Hsu TM, Law SM, Duan S, Neri BP, Kwok PY: Genotyping single-nucleotide polymorphisms by the Invader assay with dual-color fluorescence polarization detection. Clin Chem 2001, 47:1373-1377 [PubMed] [Google Scholar]
- 31.Kwok P-Y: High-throughput genotyping assay approaches. Pharmacogenomics 2000, 1:95-100 [DOI] [PubMed] [Google Scholar]


