Abstract
In delay eyeblink conditioning, the CS overlaps with the US and only a brainstem-cerebellar circuit is necessary for learning. In trace eyeblink conditioning, the CS ends before the US is delivered and several forebrain structures, including the hippocampus, are required for learning, in addition to a brainstem-cerebellar circuit. The interstimulus interval (ISI) between CS onset and US onset is perhaps the most important factor in classical conditioning, but studies comparing delay and trace conditioning have typically not matched these procedures in this crucial factor, so it is often difficult to determine whether results are due to differences between delay and trace or to differences in ISI. In the current study, we employed a 580-ms CS-US interval for both delay and trace conditioning and compared hippocampal CA1 activity and cerebellar interpositus nucleus activity in order to determine whether a unique signature of trace conditioning exists in patterns of single-unit activity in either structure. Long-Evans rats were chronically implanted in either CA1 or interpositus with microwire electrodes and underwent either delay eyeblink conditioning, or trace eyeblink conditioning with a 300-ms trace period between CS offset and US onset. On trials with a CR in delay conditioning, CA1 pyramidal cells showed increases in activation (relative to a pre-CS baseline) during the CS-US period in sessions 1-4 that was attenuated by sessions 5-6. In contrast, on trials with a CR in trace conditioning, CA1 pyramidal cells did not show increases in activation during the CS-US period until sessions 5-6. In sessions 5-6, increases in activation were present only to the CS and not during the trace period. For rats with interpositus electrodes, activation of interpositus neurons on CR trials was present in all sessions in both delay and trace conditioning. However, activation was greater in trace compared to delay conditioning in the first half of the CS-US interval (during the trace CS) during early sessions of conditioning and, in later sessions of conditioning, activation was greater in the second half of the CS-US interval (during the trace interval). These results suggest that the pattern of hippocampal activation that differentiates trace from delay eyeblink conditioning is a slow buildup of activation to the CS, possibly representing encoding of CS duration or discrimination of the CS from the background context. Interpositus nucleus neurons show strong modeling of the eyeblink CR regardless of paradigm but show a changing pattern across conditioning that may be due to the necessary contributions of forebrain processing to trace conditioning.
Keywords: Eyeblink classical conditioning, Hippocampus, Interpositus nucleus, Trace conditioning, Delay conditioning, Interstimulus interval
1. Introduction
Previous research has shown that all forms of eyeblink conditioning require a discrete brainstem-cerebellar circuit for acquisition and retention of conditioned eyeblink responses (see Christian & Thompson, 2003 for a review). In delay eyeblink conditioning, a conditioned stimulus (CS; most often a tone or a light) consistently precedes (by less than 1-sec) and overlaps with an unconditioned stimulus (US; a corneal airpuff or periorbital stimulation). Initially, only the US elicits an eyeblink response (the unconditioned response; UR). Eventually, the CS also elicits an eyeblink response (the conditioned response; CR). Delay eyeblink conditioning requires only a brainstem-cerebellar circuit for acquisition. The CA1 field of the hippocampus shows nearly immediate increases in pyramidal cell activity to the US during delay conditioning with a 250-ms CS-US interval and rapidly develops increased activity to the CS that models the amplitude-time course of the behavioral CR (Berger & Thompson, 1978a; Berger, Rinaldi, Weisz, & Thompson, 1983). However, lesions of the hippocampus do not affect (and may sometimes even enhance) delay eyeblink conditioning (Akase, Alkon, & Disterhoft, 1989; Beylin, Gandhi, Wood, Talk, Matzel, & Shors, 2001; Lee & Kim, 2004; Port, Mikhail, & Patterson, 1985; Schmaltz & Theios, 1972; Solomon & Moore, 1975), although they may have effects on retention of delay conditioning (Akase et al., 1989) or acquisition with non-optimal CS-US intervals (Beylin et al., 2001; Port et al., 1985).
In contrast to delay conditioning, a number of forms of eyeblink conditioning require the hippocampus (and other forebrain areas such as the medial prefrontal cortex) in addition to the brainstem-cerebellar areas required for delay conditioning (Christian & Thompson, 2003; Green & Woodruff-Pak, 2000). The simplest form of eyeblink conditioning that requires the hippocampus for acquisition and short-term retention is trace conditioning. In trace conditioning, the CS consistently precedes, but does not overlap with, the US. In trace eyeblink conditioning, the CS ends 250-500 ms prior to delivery of the US. Trace eyeblink conditioning appears to require the same brainstem-cerebellar circuit for acquisition and retention as delay eyeblink conditioning (Takehara, Kawahara, & Kirino, 2003; Woodruff-Pak, Lavond, & Thompson, 1985) but, when the stimulus-free trace period is long enough (250-ms for rodents; Tseng, Guan, Disterhoft, & Weiss, 2004; Weiss, Bouwmeester, Power, & Disterhoft 1999; 500-ms for rabbits, Moyer, Deyo, & Disterhoft, 1990) the hippocampus is required as well. Lesion studies have indicated that the hippocampal formation is necessary for normal acquisition and/or proper timing of trace eyeblink CRs (Beylin et al. 2001; Ivkovich & Stanton, 2001; James, Hardiman, & Yeo, 1987; Kishimoto, Nakazawa, Tonegawa, Kirino, & Kano, 2006; Moyer et al., 1990; Port, Romano, Steinmetz, Mikhail, & Patterson, 1986; Solomon, Vander Schaaf, Thompson, & Weisz, 1986; Takehara et al., 2003; Tseng et al., 2004; Weiss et al., 1999) and for short-term retention (perhaps up to several weeks; Kim, Clark, & Thompson, 1995; Takehara, Kawahara, Takatsuki, & Kirino, 2002; Takehara et al., 2003) of trace eyeblink conditioning. However, recording studies of CA1 unit activity during trace eyeblink conditioning have yielded somewhat inconsistent results. For example, it has been reported that, relative to a control group that received explicitly unpaired stimulus presentations, CA1 pyramidal cell activity in rabbits that underwent trace conditioning with a 600-ms CS-US interval (100-ms CS followed by a 500-ms trace period prior to US delivery) showed increases in activity during and immediately after the CS and after the US only during the initial blocks of trials of sessions when CRs begin to emerge (McEchron & Disterhoft, 1997). These increases in activity rapidly became smaller both within and between sessions as the CR emerged. As CRs became asymptotic, CA1 pyramidal cells showed little responsiveness to the CS and showed a decrease in firing after the US relative to unpaired stimulus presentations (McEchron & Disterhoft, 1997; Weiss, Kronforst-Collins, & Disterhoft, 1996). However, Delgado-Garcia and colleagues have reported that CA1 pyramidal cells in a group of cats that underwent trace eyeblink conditioning with a 520-ms CS-US interval (20-ms CS followed by a 500-ms trace period prior to US delivery) showed increases to CS and US onset from the beginning of CS-US presentations. Similar increases in pyramidal cell activity to CS and US onset were evident in a 500-ms delay procedure (Munera, Gruart, Munoz, Fernandez-Mas, & Delgado-Garcia, 2001), suggesting that the hippocampus may not differentiate between delay and trace conditioning at the level of single-unit activity. The amount of pyramidal cell activation to CS onset was reported to increase as delay or trace conditioning progressed. However, it is difficult to directly compare these results with those of Disterhoft and colleagues since the CS used in trace conditioning in Munera et al. (2001) was very short (20-ms) and conditioning was preceeded by extensive (240 presentations) exposure to the CS, which tends to dampen the CR-related modeling of hippocampal units during delay conditioning (Katz, Rogers, & Steinmetz, 2002).
In the current study, we sought to determine the extent to which the timing and pattern of CA1 pyramidal cell activation that develops during trace eyeblink conditioning is different from the activation that develops during a delay eyeblink conditioning procedure which differed only in the lack of a trace period between the CS and US. Trace conditioning represents perhaps the simplest form of learning that requires the hippocampus. A number of proposals have been advanced regarding why the brief gap between the CS and the US should engage the hippocampus, including filling the trace period gap (Rodriguez & Levy, 2001; Sutton & Barto, 1981), timing the relation between the CS and US (McEchron & Disterhoft, 1999), configuring CS onset and offset into a single CS (Kehoe & Weidemann, 1999), discriminating the trace period from the intertrial interval (Bolles, Collier, Bouton, & Marlin, 1978; Kaplan & Hearst, 1982; Marchand, Luck, & DiScala, 2004), and subserving the awareness that the CS predicts the US (Clark & Squire, 1998, 1999; Clark, Manns, & Squire, 2001; Manns, Clark, & Squire, 2000a, 2000b, 2002). Comparison of hippocampal single-unit activity during trace conditioning with a procedure (delay conditioning) that does not require the hippocampus and that is identical to trace conditioning except for the lack of a trace period, would be helpful in beginning to discriminate among these alternatives.
In addition, we compared hippocampal activation patterns to those of interpositus nucleus neurons in rats undergoing delay or trace eyeblink conditioning. While it is clear that the interpositus nucleus is necessary (but not sufficient) for trace eyeblink conditioning (Woodruff-Pak et al., 1985), recent studies have raised the question of whether the cerebellum processes trace eyeblink conditioning somewhat differently than delay eyeblink conditioning. Specifically, studies using mutant mice with abnormalities in cerebellar cortex have suggested that cerebellar cortex may be necessary for delay but not trace eyeblink conditioning (Kishimoto, Hirono et al., 2001; Kishimoto, Kawahara, Fujimichi, Mori, Mishina, & Kirono, 2001; Kishimoto, Kawahara, Suzuki, Mori, Mishina, & Kirono, 2001; Woodruff-Pak, Green, Levin, & Meisler, 2006). We reasoned that hippocampal processing of trace conditioning may be observable somewhere within the brainstem-cerebellar circuit necessary for all forms of eyeblink conditioning.
2. Materials and methods
2.1. Subjects
Male Long-Evans rats were purchased from Harlan (Indianapolis, IN) and housed singly upon arrival with ad libitum chow and water. The colony was maintained on a 12 hour light-dark cycle (lights on at 7 am and off at 7 pm). Rats weighed 350-375 g at the time of arrival. All testing took place during the light phase of the schedule. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont. Data from a total of 40 rats were included in this study.
2.2. Surgery
Surgery took place at least 6 days after arrival. Rats were anesthetized using 3% isoflurane in oxygen, and, using aseptic surgical procedures, each rat was surgically prepared with a 4-wire microwire electrode bundle in either the right dorsal hippocampal CA1 field or the left interpositus nucleus, a pair of electromyographic (EMG) recording wires in the left upper eyelid muscle, and a subdermal bipolar periocular stimulation electrode dorsocaudal to the left eye. In addition, a ground wire was connected to three stainless steel skull screws.
The microwire electrode bundle (NB Labs, Denison, TX or Neurolinc Corporation, New York, NY) was constructed from four 50-μm Teflon-coated stainless steel wires soldered to a mini-strip connector. Immediately prior to implantation, the wires were cut to length with tungsten-carbide scissors, bringing the tip impedance of each wire to approximately 200-400 kOhms (at 1 kHz). Implantation was in either the right hippocampal CA1 field (AP: -3.8 from bregma; ML: -1.6; DV: -2.7 to -3.2), or the left interpositus nucleus (AP: -2.4 from the interaural line; ML: +2.4; DV: -5.8). Final electrode depth for the hippocampal CA1 field was determined by monitoring unit activity during implantation. The mini-strip connector was cemented to the skull with dental cement.
The EMG wires for recording activity of the external muscles of the eyelid, the orbicularis oculi, were constructed from two strands of 75-μm Teflon-coated stainless steel wire soldered at one end to a mini-strip connector. The other end of each wire was passed subdermally to penetrate the skin of the upper eyelid of the left eye. The bipolar stimulation electrode (Plastics One, Roanoke, VA) was positioned subdermally immediately dorsocaudal to the left eye. The mini-strip connector and the bipolar stimulation electrode were cemented to the skull with dental cement. The wound was salved with antibiotic ointment (Povidone), and an analgesic (buprenorphine) was administered (s.c.) immediately after surgery and twice the following day. Rats were given 6-7 days to recover prior to eyeblink conditioning.
2.3. Apparatus
Eyeblink conditioning took place in one of four identical testing chambers (30.5 × 24.1 × 29.2 cm; Med-Associates, St. Albans, VT), each with a grid floor. The top of each chamber was modified so that a 25-channel tether/commutator could be mounted to it. Each testing chamber was housed within a separate electrically-shielded, sound-attenuating chamber (45.7 × 91.4 × 50.8 cm; BRS-LVE, Laurel, MD). A fan in each sound-attenuating chamber provided background noise of approximately 60 dB sound pressure level. A speaker was mounted in each corner of the rear wall and a houselight (off during testing) was mounted in the center of the rear wall of each sound-attenuating chamber. The sound-attenuating chambers were housed within a walk-in sound-proof chamber.
Stimulus delivery was controlled by an IBM PC-compatible computer running custom software (Chen & Steinmetz, 1998). A 2800 Hz, 80 dB tone, delivered through the left speaker of the sound-attenuating chamber, served as the conditioned stimulus (CS). The CS was 590-ms in duration for delay conditioning and 280-ms in duration for trace conditioning. A 10 ms, 60 Hz, 4.0 mA uniphasic periorbital stimulation, delivered from a constant current stimulator (model A365D; World Precision Instruments, Sarasota, FL), served as the unconditioned stimulus (US) during conditioning. Recording of eyelid EMG activity and neural activity was controlled by a computer interfaced with a Power 1401 high-speed data acquisition unit and running Spike2 software (CED, Cambridge, UK). The eyelid EMG signal was amplified (10k) and bandpass filtered (100-1000 Hz) and each neural signal was passed through a JFET configured as a source follower, amplified (10k) and bandpass filtered (600-6000 Hz) prior to being passed to the Power 1401 and from there to a computer running Spike2. Sampling rate was 2 kHz for EMG activity and 20 kHz for each channel of neural activity. The Spike2 software was used to full-wave rectify, smooth (10 ms time constant), and time shift (10 ms, to compensate for smoothing) the amplified EMG signal to facilitate behavioral data analysis.
2.4. Conditioning Procedures
Beginning 6-7 days after surgery, rats underwent eyeblink conditioning. For each day of conditioning, the rat was plugged in, via the connectors cemented to its head, to the 25-channel tether/commutator, which carried leads to and from peripheral equipment and allowed the rat to move freely within the testing box. On Day 1 (adaptation), rats were plugged in but no stimuli were delivered. They remained in the chamber for 60-min (the approximate length of a training session). On Days 2-7 (conditioning), rats were given 100 trials each day, at an average intertrial interval (ITI) of 30-sec (range = 20-40 sec). Two groups of rats received trials consisting of a 590-ms tone CS which coterminated with a 10-ms, 4-mA US (580-ms delay paradigm), except for every tenth trial, in which the CS was presented alone (for inspection of potential long-latency responses). The other two groups of rats received trials consisting of a 280-ms tone CS, a 300-ms trace period, and a 10-ms, 4-mA US (580-ms trace paradigm), except for every tenth trial, in which the CS was presented alone.
2.5. Histology
One or two days after conditioning was completed, rats were overdosed with sodium pentobarbital (150 mg/kg) and transcardially perfused with 0.9% saline followed by 10% buffered formalin. A small dc electrolytic lesion (100 μA, 10 sec) was made by passing current through one of the unit recording electrodes. The brain was removed and stored in 10% buffered formalin. Three or four days prior to sectioning, the brain was transferred to a 30% sucrose/10% buffered formalin solution. Before sectioning, brains that had a cerebellar implant were embedded in albumin-gelatin. Frozen sections were taken at 90 μm. The tissue was mounted on gelatin-coated glass slides, stained with cresyl violet (for cell bodies) and Prussian blue (for iron deposits left by the marking lesions) and coverslipped with Permount.
2.6. Behavioral Data Analysis
For conditioning sessions, trials were subdivided into four time periods: (1) a “baseline”period, 280-ms prior to CS onset; (2) a non-associative “startle” period, 0-80 ms after CS onset; (3) a “CR” period, 81-580 ms after CS onset; and (4) a “UR period”, 0-280 ms after US onset. Eye blinks that exceeded mean baseline activity by 0.5 arbitrary units during the CR period were scored as CRs. Eye blinks that met this threshold during the startle period were scored as startle responses (SRs). The dependent measures of CR acquisition were percentage of CRs and eyeblink response magnitude across all CS-US trials of each session.
2.7. Unit Data Analysis
Offline separation of individual units was done using Spike2. An initial amplitude threshold of at least two times baseline noise and an initial temporal window of 1.1 ms (0.5 ms before the peak of a spike to 0.6 ms after) was set to pick out potential units. For each electrode with discriminable units, principal components analysis based on spike waveforms was followed by cluster analysis, using K means clustering (number of clusters = 7; match to classes within 2-3 SDs), for initial separation of waveforms into those from different units. Careful inspection was then made of the waveforms of a sample of individual units and cluster boundaries were adjusted as needed. Typically, one or two electrodes per rat yielded well-isolated units in each session. For each of these electrodes, 1-2 units could be separated and very occasionally up to 5 units could be isolated. Although it is possible that a single unit was isolated and recorded during more than one session, we assumed that this was not the case. Rather, separated units were treated as independent events across training for each rat. For hippocampal CA1 recordings, units with an average spontaneous rate of firing of 8 spikes/sec or less (measured in the 500-ms prior to CS onset) were classified as pyramidal cells while those with an average spontaneous rate of firing of more than 8 spikes/sec were classified as interneurons (Fox & Ranck, 1981; Ranck, 1973).
Following spike separation, behavioral and unit data were binned (bin size = 0.5 ms) and analyzed using custom software (King & Tracy, 1999). Data were separated according to trial type (CS-alone, CS-US) and behavior type (CR, non-CR) and analyzed independently. For analyses of increases and decreases in neuron firing during trials, the time between CS onset and US onset and the 290-ms after US onset was divided into subperiods of 58-ms each. This resulted in ten CS periods and five US periods. For each trial, difference scores were calculated by subtracting the mean activity for the 500-ms prior to CS onset from the mean activity for each of the post-CS and post-US periods. For a session, ten CS period standard scores and five US period standard scores were formed by dividing the mean of the corresponding difference score by the standard error of the corresponding difference score. The first US period (0-60 ms after the onset of the 10-ms US) was not further analyzed due to the presence of US-related artifact. Criterion for a significant increase or decrease in activity in a particular subperiod was 2.0 standard scores.
3. Results
3.1. Histology
Figure 1 depicts the locations of electrodes that yielded discriminable units from the hippocampal CA1 field (Figure 1A) and the cerebellar interpositus nucleus (Figure 1B). Stimulation (20-100 μA) delivered through interpositus nucleus electrodes was able to elicit eyelid EMG activity.
Figure 1.

Microwire bundle placements (shaded area). (A) Hippocampal CA1 pyramidal cell layer recording electrodes. (B) Cerebellar interpositus nucleus recording electrodes.
3.2. Eyeblink Conditioning
Figure 2A depicts the percentage of CRs and eyeblink response magnitude on CS-US trials across the six sessions of conditioning for rats that underwent delay conditioning (n=19) and rats that underwent trace conditioning (n=21). As can be seen, delay and trace conditioning were acquired at similar rates (inspection of CS-alone trials also did not reveal any differences in responding between delay and trace procedures), which has been observed previously when trace conditioning with a short trace interval (less than 500-ms in eyeblink conditioning; less than 5-sec in fear conditioning) is compared to delay conditioning matched for CS-US interval (see Discussion). These observations were confirmed with a 2 (Group: Delay, Trace) × 6 (Session) repeated-measures analysis of variance (ANOVA) conducted on percentage of CRs. Only the main effect of session was significant, F (5, 190) = 17.02, p < 0.01. A similar repeated-measures ANOVA conducted on response magnitude also revealed only a significant main effect of session, F (5, 190) = 15.89, p < 0.01. Figures 2B-D depict histograms showing CR peak latencies of all CRs on paired trials for delay and trace conditioning across sessions. Average topography of CRs was similar for delay and trace conditioning.
Figure 2.

Percentage of eyeblink CRs, eyeblink response magnitude, and eyeblink CR topography during conditioning. (A) Percentage of CRs (left) and eyeblink response magnitude (right) as a function of session for rats that underwent 580-ms delay conditioning or 580-ms trace conditioning with a 300-ms trace interval. (B) CR peak latency distribution as a function of 58-ms subperiod during the CS-US interval in conditioning sessions 1 and 2. (C) CR peak latency distribution as a function of 58-ms subperiod during the CS-US interval in conditioning sessions 3 and 4. (D) CR peak latency distribution as a function of 58-ms subperiod during the CS-US interval in conditioning sessions 5 and 6.
3.3. Hippocampal CA1 Pyramidal Cell Activity
Offline separation of multiple-unit recordings yielded a total of 273 single units that were classified as pyramidal cells based on a spontaneous firing rate (measured in the 500-ms prior to CS onset) of 8 spikes/sec or less (average of 4.15 ± 0.15 spikes/sec).
Pyramidal cell activity was analyzed during pairs of sessions. A total of 113 units classified as CA1 pyramidal cells were recorded from rats undergoing delay conditioning (sessions 1-2, 38 units; sessions 3-4, 43 units; sessions 5-6, 32 units), and a total of 160 units classified as CA1 pyramidal cells were recorded from rats undergoing trace conditioning (sessions 1-2, 72 units; sessions 3-4, 42 units; sessions 5-6, 46 units). Activation patterns during the CS-US interval and during the interval 61-290 ms after US onset were examined in terms of increases and decreases in firing relative to a pre-CS baseline on trials with a CR compared to trials without a CR. Results for the 10 interspersed CS-alone trials were not analyzed because there were too few trials of this type.
Population excitation during the CS-US interval on CR trials was present from the beginning of conditioning in animals undergoing delay conditioning. The average activation level declined somewhat across sessions, and some inhibition on non-CR trials began to emerge in later sessions. Population excitation after the US was observed from the beginning of conditioning independent of the presence or absence of CRs. This excitation remained elevated across conditioning for trials in which a CR had been emitted but declined for non-CR trials. Figure 3 depicts average standard scores for all CA1 pyramidal cells recorded during delay conditioning. Figure 4 depicts an example of a CA1 pyramidal cell that showed excitation on CR trials in session 2 of delay conditioning.
Figure 3.

Mean standard scores for CA1 pyramidal cells recorded during 580-ms delay conditioning as a function of 58-ms subperiod during and after the CS-US interval for CR trials and non-CR trials. (A) Sessions 1 and 2. (B) Sessions 3 and 4. (C) Sessions 5 and 6.
Figure 4.

An example of a CA1 pyramidal cell recorded during session 2 of delay conditioning that showed an increase in activity during the CS-US interval on trials with a CR. (A) Amplified, rectified, and integrated eyelid EMG activity from a trial (top trace), unit activity from a trial (middle trace), and tick marks representing the discriminated unit summarized in C. (B) Mean eyelid activity across blocks of trials. (C) Mean standard scores as a function of 58-ms subperiod during and after the CS-US interval for CR trials.
In contrast to animals undergoing delay conditioning, population excitation of CA1 pyramidal cells during the CS-US interval on CR trials was slow to develop in animals undergoing trace conditioning. More noticeable during early sessions of trace conditioning was inhibition of CA1 pyramidal cells on trials without a CR. However, by Sessions 5-6, population activation during the CS portion of the CS-US interval on trials with a CR had increased in trace conditioned animals. Population activation during the trace period remained low on trials without a CR. Thus, by the end of conditioning, patterns of CA1 pyramidal cell activity in animals undergoing trace conditioning looked similar to those of animals undergoing delay conditioning during the first half of the CS-US interval, when the tone CS was on for both groups, but continued to look different during the second half of the CS-US interval, when the tone CS was off for the animals undergoing trace conditioning.
Figure 5 depicts average standard scores for all CA1 pyramidal cells recorded during trace conditioning. Figure 6 depicts a CA1 pyramidal cell that showed inhibition on non-CR trials during session 1 of trace conditioning. Figure 7 depicts the changing proportions of significantly (absolute standard score of 2.0 or greater) excited and inhibited CA1 pyramidal cells during the CS-US interval and the post-US period on CR trials from animals undergoing delay and trace conditioning. This figure illustrates the shift in trace conditioned animals from more cells showing inhibition during the CS-US interval in early sessions to more cells showing excitation to the CS in later sessions. In contrast, cells showing excitation predominated during the CS-US interval in delay conditioning throughout all sessions.
Figure 5.

Mean standard scores for CA1 pyramidal cells recorded during 580-ms trace conditioning with a 300-ms trace period as a function of 58-ms subperiod during and after the CS-US interval for CR trials and non-CR trials. (A) Sessions 1 and 2. (B) Sessions 3 and 4. (C) Sessions 5 and 6.
Figure 6.

An example of a CA1 pyramidal cell recorded during session 1 of trace conditioning that showed a decrease in activity during the CS-US interval on trials with no CR. (A) Amplified, rectified, and integrated eyelid EMG activity from a trial (top trace), unit activity from a trial (middle trace), and tick marks representing the discriminated unit summarized in C. (B) Mean eyelid activity across blocks of trials. (C) Mean standard scores as a function of 58-ms subperiod during and after the CS-US interval for CR trials.
Figure 7.

Proportion of CA1 pyramidal cells showing significant (absolute standard score of 2.0 or above) excitation or inhibition on CR trials during delay or trace conditioning as a function of period during trials. CS period represents the first half (0-290 ms after CS onset) of the CS-US interval. CS/trace period represents the second half (291-580 ms after CS onset) of the CS-US interval, when the CS was either still on (delay) or was off (trace). US period represents the period after the US was delivered and the stimulation artifact was no longer present (61-290 ms after US onset).
Observations of patterns of CA1 pyramidal cell activity during the CS-US interval were confirmed with three 2 (Group: Delay, Trace) X 2 (Behavior Type: CR Trial, non-CR Trial) X 10 (CS Periods 1-10) repeated-measures ANOVAs conducted on standard scores for Sessions 1-2, Sessions 3-4, and Sessions 5-6. Analysis of standard scores for Sessions 1-2 revealed a significant CS Period by Group interaction effect, F (9, 963) = 2.87, p < 0.01 and a significant Behavior Type by CS Period interaction effect, F (9, 963) = 9.54, p < 0.01. Further analysis of the CS Period by Group interaction effect with a series of one-way ANOVAs comparing Groups within each CS Period (collapsed across Behavior Types) revealed significantly higher standard scores for delay conditioning in CS Periods 2, 4, 5, 6, 7, 9, and 10 (p’s < 0.05). Analysis of standard scores for Sessions 3-4 revealed a significant Behavior Type by CS Period interaction effect, F (9, 756) = 4.33, p < 0.01 and a significant Group main effect, F (1, 84) = 7.62, p < 0.01. Standard scores for animals undergoing delay conditioning were significantly higher in all CS Periods compared to animals undergoing trace conditioning. Analysis of standard scores for Sessions 5-6 revealed a significant CS Period by Group interaction effect, F (9, 684) = 3.44, p < 0.01 and a significant Behavior Type by CS Period interaction effect, F (9, 684) = 3.67, p < 0.01. Further analysis of the CS Period by Group interaction effect with a series of one-way ANOVAs comparing Groups within each CS Period (collapsed across Behavior Types) revealed significantly higher standard scores for trace conditioning in CS Periods 1 and 5 (p’s < 0.05), which correspond to the onset and offset of the trace CS.
Observations of patterns of CA1 pyramidal cell activity after the US were confirmed with three 2 (Group: Delay, Trace) X 2 (Behavior Type: CR Trial, non-CR Trial) X 4 (US Periods 12-15) repeated-measures ANOVAs conducted on standard scores for Sessions 1-2, Sessions 3-4, and Sessions 5-6. Analysis of standard scores for Sessions 1-2 revealed a significant US Period by Group interaction effect, F (3, 321) = 2.84, p < 0.05. There were no differences in standard scores between CR and non-CR trials. Further analysis of the US Period by Group interaction effect with a series of one-way ANOVAs comparing Groups within each US Period (collapsed across Behavior Types) revealed significantly higher standard scores for delay conditioning in US Periods 14 and 15 (p’s < 0.02). Analysis of standard scores for Sessions 3-4 revealed a significant US Period by Group interaction effect, F (3, 252) = 3.00, p < 0.05 and a significant Behavior Type main effect, F (1, 84) = 10.25, p < 0.01. Further analysis of the US Period by Group interaction effect with a series of one-way ANOVAs comparing Groups within each US Period (collapsed across Behavior Types) revealed significantly higher standard scores for delay conditioning in Periods 13, 14, and 15 (p’s < 0.04). Analysis of standard scores for Sessions 5-6 revealed a significant US Period by Group interaction effect, F (3, 228) = 10.51, p < 0.01 and a significant Behavior Type main effect, F (1, 76) = 16.55, p < 0.01. Further analysis of the US Period by Group interaction effect with a series of one-way ANOVAs comparing Groups within each US Period (collapsed across Behavior Types) revealed significantly higher standard scores for delay conditioning in US Periods 14 and 15 (p’s < 0.05). However, for US Period 12, animals that underwent trace conditioning had a significantly higher standard scores (p = 0.05).
3.4. Hippocampal CA1 Interneuron Activity
Not enough CA1 interneurons, defined as units with an average baseline firing rate of greater than 8 spikes/sec, were recorded for systematic analyses of behavior-related firing patterns. Either excitation or inhibition during the CS-US interval on CR trials was observed in individual CA1 interneurons in both delay and trace conditioning.
3.5. Cerebellar Interpositus Nucleus Activity
Offline separation of multiple-unit recordings yielded a total of 187 units. Mean spontaneous firing rate (measured in the 500-ms prior to CS onset) of these units was 26.10 ± 1.21 spikes/sec.
Interpositus nucleus unit activity was analyzed during pairs of sessions. A total of 87 units were recorded from rats undergoing delay conditioning (sessions 1-2, 32 units; sessions 3-4, 31 units; sessions 5-6, 24 units), and a total of 100 units were recorded from rats undergoing trace conditioning (sessions 1-2, 40 units; sessions 3-4, 34 units; sessions 5-6, 26 units). Activation patterns during the CS-US interval and during the interval 61-290 ms after US onset were examined in terms of increases and decreases in firing relative to a pre-CS baseline on trials with a CR compared to trials without a CR. Results for the 10 interspersed CS-alone trials were not analyzed because there were too few trials of this type.
Population activation during the CS-US interval on CR trials was present from the beginning of conditioning in all animals, regardless of whether they were undergoing delay or trace conditioning. For early sessions of delay conditioning, population activation was more prominent the closer the period was to US onset and this pattern remained present throughout conditioning. Figure 8 depicts average standard scores for all interpositus nucleus neurons recorded during delay conditioning.
Figure 8.

Mean standard scores for cerebellar interpositus neurons recorded during 580-ms delay conditioning as a function of 58-ms subperiod during and after the CS-US interval for CR trials and non-CR trials. (A) Sessions 1 and 2. (B) Sessions 3 and 4. (C) Sessions 5 and 6.
Animals undergoing trace conditioning showed a more nuanced pattern of population activation. Specifically, in Sessions 1-2, regardless of whether a CR was emitted or not, there was greater interpositus activation during the CS in trace conditioning animals, comared to similar periods (i.e., the first half of the CS-US interval) in delay conditioning animals. In Sessions 3-4, on CR but not non-CR trials, there was greater interpositus activation during the CS and the early portions of the trace period in trace conditioning animals compared to similar periods in delay conditioning animals. Finally, in Sessions 5-6, the pattern evident in Sessions 3-4 had shifted to later in the CS-US interval. Specifically, on CR but not on non-CR trials, there was greater interpositus activation during the trace period in trace conditioning animals compared to similar periods in delay conditioning animals. Figure 9 depicts average standard scores for all interpositus nucleus neurons recorded during trace conditioning. Figure 10 depicts an interpositus nucleus neuron that showed excitation on CR trials in trace conditioning.
Figure 9.

Mean standard scores for cerebellar interpositus neurons recorded during 580-ms trace conditioning with a 300-ms trace period as a function of 58-ms subperiod during and after the CS-US interval for CR trials and non-CR trials. (A) Sessions 1 and 2. (B) Sessions 3 and 4. (C) Sessions 5 and 6.
Figure 10.
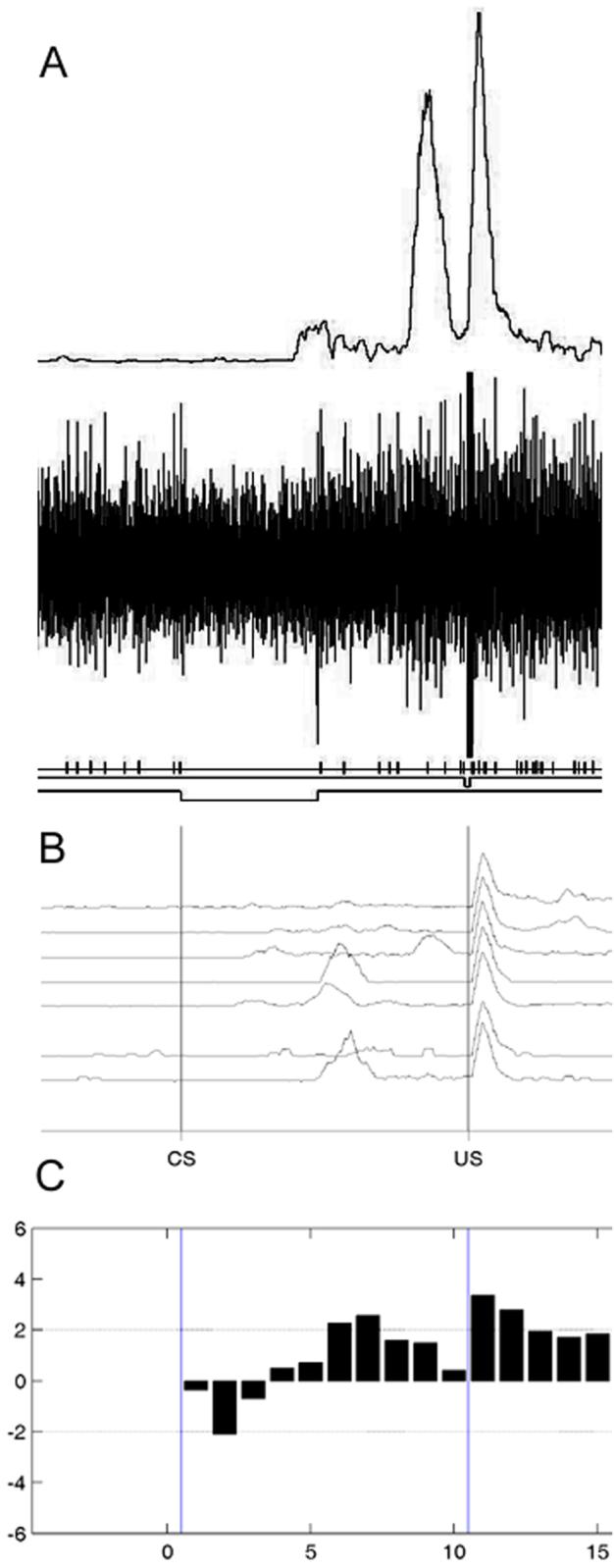
An example of a cerebellar interpositus neuron recorded during trace conditioning that showed an increase in activity during the CS-US interval on trials with a CR. (A) Amplified, rectified, and integrated eyelid EMG activity from a trial (top trace), unit activity from a trial (middle trace), and tick marks representing the discriminated unit summarized in C. (B) Mean eyelid activity across blocks of trials. (C) Mean standard scores as a function of 58-ms subperiod during and after the CS-US interval for CR trials.
Population activation after the US was present on both CR trials and non-CR trials in both delay and trace conditioned animals. Early in conditioning, activation after the US was greater on non-CR trials compared to CR trials but was similar for delay and trace conditioning. Across later sessions, interpositus activation in the post-US period remained more or less consistent for delay conditioning animals but showed a shift in trace conditioning animals, with interpositus activation being similar for CR and non-CR trials in the middle sessions and then increasing on CR trials compared to non-CR trials in later sessions.
Observations of patterns of interpositus nucleus neuron activity during the CS-US interval were confirmed with three 2 (Group: Delay, Trace) X 2 (Behavior Type: CR Trial, non-CR Trial) X 10 (CS Periods 1-10) repeated-measures ANOVAs conducted on standard scores for Sessions 1-2, Sessions 3-4, and Sessions 5-6. Analysis of standard scores for Sessions 1-2 revealed a significant CS Period by Group interaction effect, F (9, 630) = 4.52, p < 0.01 and a significant Behavior Type by CS Period interaction effect, F (9, 630) = 18.01, p < 0.01. Further analysis of the CS Period by Group interaction effect with a series of one-way ANOVAs comparing Groups within each CS Period (collapsed across Behavior Types) revealed significantly higher standard scores for trace conditioning in CS Periods 2-5 (p’s < 0.05). Analysis of standard scores for Sessions 3-4 revealed a significant CS Period by Group interaction effect, F (9, 567) = 2.30, p < 0.02, a significant Behavior Type by Group interaction effect, F (1, 63) = 6.61, p < 0.02, and a significant Behavior Type by CS Period interaction effect, F (9, 567) = 30.91, p < 0.01. Further analysis of the CS Period by Group interaction effect with a series of one-way ANOVAs comparing Groups within each CS Period (collapsed across Behavior Types) revealed significantly higher standard scores for trace conditioning in CS Periods 1-3, and 5 (p’s < 0.05) (i.e., during the CS for trace conditioning). Further analysis of the Behavior Type by Group interaction effect with a series of one-way ANOVAs comparing Groups within each Behavior Type (collapsed across CS Periods) revealed significantly higher standard scores for trace conditioning on CR trials (p < 0.01) but no difference between delay and trace on non-CR trials. Analysis of standard scores for Sessions 5-6 revealed a significant Behavior Type by CS Period by Group interaction effect, F (9, 432) = 2.69, p < 0.01. Further analysis of the three-way interaction effect with a series of one-way ANOVAs comparing Groups within each Behavior Type and CS Period revealed significantly higher standard scores for trace conditioning on CR trials in CS Periods 2 and 5-10 (p’s < 0.03) (i.e., mostly during the trace interval) and a significant lower standard score for trace conditioning on non-CR trials in period 6 (p < 0.02).
Observations of patterns of interpositus nucleus neuron activity after the US were confirmed with three 2 (Group: Delay, Trace) X 2 (Behavior Type: CR Trial, non-CR Trial) X 4 (US Periods 12-15) repeated-measures ANOVAs conducted on standard scores for Sessions 1-2, Sessions 3-4, and Sessions 5-6. Analysis of standard scores for Sessions 1-2 revealed a Behavior Type by US Period interaction effect, F (3, 210) = 7.06, p < 0.01. There were no differences in standard scores between groups. Analysis of standard scores for Sessions 3-4 revealed a Behavior Type by US Period by Group interaction effect, F (3, 189) = 4.77, p < 0.01. Further analysis of the three-way interaction effect with a series of one-way ANOVAs comparing Groups within each Behavior Type and US Period revealed significantly higher standard scores for trace conditioning on non-CR trials in US Periods 13-15 (p’s < 0.02). Analysis of standard scores for Sessions 5-6 revealed a Behavior Type by US Period by Group interaction effect, F (3, 144) = 4.33, p < 0.01. Further analysis of the three-way interaction effect with a series of one-way ANOVAs comparing Groups within each Behavior Type and US Period revealed significantly higher standard scores for trace conditioning on CR trials in US Periods 12-14 (p’s < 0.01) and a significantly higher standard score for delay conditioning on non-CR trials in US Period 14 (p < 0.03).
4. Discussion
The results of this study can be summarized as follows: (1) Hippocampal CA1 pyramidal cell activation to the CS was slow to develop in trace eyeblink conditioning compared to a delay eyeblink conditioning procedure matched in terms of CS-US interval. Population activation during delay conditioning occurred across the CS-US interval, when CRs were emitted, from the beginning of conditioning and continued throughout conditioning. When activation did develop in later sessions of trace conditioning, it occurred during the CS, when CRs were emitted, but not during the trace period; (2) Hippocampal CA1 pyramidal cell inhibition during non-CR trials was observed in trace conditioning in early sessions, and in both delay and trace conditioning in later sessions; (3) Hippocampal CA1 pyramidal cell activation to the US tended to be fairly similar early in delay and trace conditioning and was not tied to the presence or absence of CRs. As conditioning progressed, activation to the US tended to become more robust on CR trials, particularly in delay conditioning; (4) Cerebellar interpositus nucleus neurons showed activation that was strongly tied to the temporal pattern of conditioned eyeblinks regardless of paradigm. Animals undergoing delay conditioning showed a model of the learned eyeblink response that changed very little across conditioning. In contrast, the pattern of activation of interpositus neurons from animals undergoing trace conditioning gradually shifted from being more prominent during the CS (compared to equivalent time periods for animals undergoing delay conditioning) to more prominent during the trace period (compared to equivalent time periods for animals undergoing delay conditioning).
Because we did not include unpaired control groups, we cannot unequivocally rule out non-associative processes playing a role in the different patterns of hippocampal CA1 and interpositus nucleus activation and inhibition during delay and trace conditioning that we observed in this study. However, it is unlikely that non-associative processes played a major (or any role) in the differences we observed. The only way a non-associative explanation could account for our results would be to show that pseudoconditioning with a short tone (as was used in trace conditioning) produces activation patterns that differ from pseudoconditioning with a longer tone (as was used in delay conditioning), such as a slower buildup of hippocampal CA1 pyramidal cell activation to shorter tones. However, studies have consistently shown little or no activation of hippocampal CA1 pyramidal cells or interpositus nucleus neurons to the tone during unpaired presentations of a tone and eye stimulation (although there may be activation to the eye stimulation). For example, McEchron and Disterhoft (1997) showed that unpaired tone and airpuff presentations produced little or no hippocampal CA1 pyramidal cell activation to the tone compared to trace conditioning. In addition, Berger et al. (1983) reported the same results for unpaired stimulus presentations compared to short delay conditioning and we have observed little or no activation of interpositus nucleus neurons to the tone during unpaired tone and periorbital stimulation compared to delay conditioning (Green, Johnson, Goodlett, & Steinmetz, 2002).
We used a trace conditioning procedure with a short (300-ms) trace interval, which has been shown to be hippocampally-dependent in rats and mice (Tseng et al., 2004; Weiss et al., 1999), in order to produce a rate of learning that was not substantially slower than the rate of learning of delay conditioning. Animals that underwent delay conditioning showed somewhat poorer learning in the current study than in previous studies using the same parameters in our lab (unpublished observations). However, equivalent rates of delay and trace conditioning allowed us to conclude that the level of conditioning cannot account for the different patterns of neural activity during delay and trace conditioning. It should be noted that equivalent rates of delay and trace conditioning and equivalent asymptotes, when the trace interval is short, have been reported by others. For example, Kehoe and Napier (1991), using rabbits, found similar rates of 400-ms delay and 400-ms trace eyeblink conditioning with a 300-ms trace interval and Burman and Gewirtz (2004) reported equivalent fear after 7-sec delay and 7-sec trace fear conditioning with a 3-sec trace interval when fear was assessed using fear-potentiated startle.
4.1. The Involvement of the Hippocampal CA1 Field in Trace Conditioning
The results of previous unit recording studies in rabbits have suggested a rapid engagement of CA1 pyramidal cells during 250-ms delay eyeblink conditioning (Berger et al., 1983) and a slower engagement of CA1 pyramidal cells during 600-ms trace eyeblink conditioning with a 500-ms trace period (McEchron & Disterhoft, 1997). However, it was unclear whether these differences reflected differences in the CS-US interval or the presence versus the absence of a trace period. In the only published study to match CS-US intervals and record from CA1 pyramidal cells during delay and trace eyeblink conditioning, CA1 pyramidal cell activation developed primarily to CS and US onset during both delay and trace eyeblink conditioning in the cat, and increased in magnitude to CS onset as conditioning progressed. However, an unusually short CS (20-ms) was used during trace conditioning and animals were given extensive (240 trials) CS preexposure (Munera et al., 2001), so the generality of these results for typical delay and trace conditioning remained in question. For example, CS preexposure attenuates CR-related hippocampal CA1 activation during subsequent conditioning (Katz, Rogers, & Steinmetz, 2002). Given the fact that large hippocampal lesions impair trace eyeblink conditioning (Ivkovich & Stanton, 2001; Kishimoto et al., 2006; Moyer et al., 1990; Solomon et al., 1986; Tseng et al., 2004; Weiss et al., 1999) but not delay eyeblink conditioning (Akase et al., 1989; Lee & Kim, 2004; Port et al. 1985; Schmaltz & Theios, 1972; Solomon & Moore, 1975), even when delay and trace are matched for CS-US interval (Beylin et al., 2001; Ivkovich & Stanton, 2001), it might be expected that the development and pattern of hippocampal activation during trace conditioning would appear different from the development and pattern of hippocampal activation during delay conditioning when they are matched for CS-US interval. Resolution of this issue might provide further clues as to the type of information that the hippocampus is communicating to the cerebellum (or vice versa) during different phases of training which allows trace conditioning to occur but which is not necessary for delay conditioning. Our results suggest that the hippocampus is processing the CS regardless of paradigm. In addition, our results suggest that it may take longer for the hippocampus to respond to the stimulus serving as the CS in trace conditioning compared to delay conditioning. These results are fairly congruent with the findings of McEchron and Disterhoft (1997) for trace conditioning in the restrained rabbit preparation, with the main difference being that, in our study using freely-moving rats, activation to the CS occurred after some CRs had already developed whereas McEchron and Disterhoft reported that activation to the CS occurred only as CRs began to emerge. It is possible that these differences in activation patterns are due to differences between eyeblink conditioning in freely-moving rats compared to restrained rabbits or due to differences in the way eyeblinks were measured and scored as CRs. In any case, our results extend these previous results by showing that this pattern of activation is a specific marker of trace conditioning and does not occur in a delay conditioning procedure matched for CS-US interval.
Our results, and those of others, raise the question of why hippocampal CA1 pyramidal cells take so long to become engaged by a procedure in which they appear to be necessary (trace conditioning) yet become engaged rapidly in a procedure in which they are not necessary (delay conditioning). One possibility is that the hippocampus allows discrimination of the CS from the background context as being predictive of the US. In delay conditioning, this occurs almost immediately, since the US is present only when the CS is also present and not when just the background context is present. In trace conditioning, this discrimination requires a number of trials, since the background context is present by itself when the US is present and the longer the trace interval, the more difficult this discrimination is to make. Thus, in trace conditioning, the animal must learn that the CS, and not the background context, is predictive of the US. The current results suggest that hippocampal CA1 pyramidal cells likely play a role in this discrimination. Disterhoft and colleagues have shown that single units in caudal medial prefrontal cortex respond to the trace CS from the earliest sessions of eyeblink conditioning, unlike single units in CA1 (Weible, Weiss, & Disterhoft, 2003). They speculated that these units may provide information to the hippocampus regarding the behavioral salience of the CS, which is why hippocampal units are slow to develop activation to the CS in trace conditioning. It should be noted that the current results, as well as previous results from both trace eyeblink conditioning (e.g., McEchron & Disterhoft, 1997; Munera et al., 2001) and trace fear conditioning (e.g., Gilmartin & McEchron, 2005a) do not support the hypothesis that hippocampal CA1 pyramidal cells serve to “bridge the gap” between the CS and the US for trace eyeblink conditioning to occur, at least not in the sense of sustained activation of units that is initiated by the CS and persists until US delivery. It may be either that this function is mediated by another subregion of the hippocampus or another brain area, such as the anterior cingulate cortex in trace eyeblink conditioning (Weible et al., 2003) or the prelimbic region of the medial prefrontal cortex in trace fear conditioning (Gilmartin & McEchron, 2005b) or that “bridging the gap” is not critical for trace conditioning to occur. In support of this latter notion are findings that are not easily explained by a “gap filler” account of trace conditioning. For example, trace eyeblink conditioning with very long trace intervals (3-sec) can be produced by decreasing the number of trials per session (Kehoe, Cool, & Gormezano, 1991) and trace fear conditioning can be facilitated by a post-US stimulus that serves to distinguish the trace interval from early portions of the intertrial interval (Bolles et al., 1978).
4.2. The Involvement of the Cerebellar Interpositus Nucleus in Trace Conditioning
While it has been shown previously that the interpositus nucleus is critical for trace eyeblink conditioning (Woodruff-Pak et al., 1985), the possibility that it may be engaged in trace conditioning differently from delay conditioning has not been widely explored. To our knowledge, the only published study which recorded from interpositus nucleus neurons during trace eyeblink conditioning showed that, in the cat, activation or inhibition of neurons occurred after the onset of CRs in both delay and trace conditioning (Gruart, Guillazo-Blanch, Fernandez-Mas, Jimenez-Diaz, & Delgado-Garcia, 2000). However, in this study, a very short (20-ms), weak airpuff was used as the CS in trace conditioning, and animals were given extensive (240 trials) CS preexposure, so it was unclear if these results would generalize to a more usual trace conditioning procedure. In addition, these researchers recorded from posterior interpositus nucleus in the cat while previous single-unit studies of delay eyeblink conditioning in the rabbit and the rat have recorded from anterior interpositus nucleus neurons and have reported activation that precedes and coincides with the eyeblink CR (Berthier & Moore, 1990; Freeman & Nicholson, 1999; McCormick & Thompson, 1984; Nicholson & Freeman, 2002; Rogers, Britton, & Steinmetz, 2001). Our results suggest that the activation patterns of anterior interpositus nucleus neurons during trace eyeblink conditioning are closely tied to the presence of the behavioral CR, as in delay eyeblink conditioning. However, relatively greater activation during the early portions of the CS-US interval in trace conditioning (when the CS is present) compared to delay conditioning in early sessions of conditioning and a shift in this pattern to later portions of the CS-US interval (during the trace period) in later sessions of conditioning may reflect the contribution of forebrain structures to trace conditioning.
Proposals regarding how forebrain areas influence cerebellar processing during eyeblink conditioning have centered on the notion that this influence occurs via inputs from forebrain areas to the pontine nuclei (Berger & Bassett, 1992; Berger, Berry, & Thompson, 1986; Katz & Steinmetz, 2002). Since the pontine nuclei provide CS-related information to the cerebellum (Steinmetz, Logan, Rosen, Thompson, Lavond, & Thompson, 1987), these forebrain inputs would be expected to influence CS processing. The hippocampus does not have a strong monosynaptic connection with the pontine nuclei, but it may exert an influence on the pontine nuclei via its connections with medial prefrontal cortex, retrosplenial cortex, or mammillary nuclei. Each of these regions has direct projections to the pontine nuclei in the rat (Ruigrok, 2004). Medial prefrontal cortex is currently the most likely candidate, since lesions confined to the retrosplenial cortex do not affect trace conditioning (Weible et al., 2000), and an early recording study of limbic system involvement in delay eyeblink conditioning failed to find eyeblink CR-related activity in the mammillary nuclei (Berger & Thompson, 1978b). Medial prefrontal cortex in the rat receives prominent projections from both CA1 and the subiculum and projects back to the hippocampal formation via the entorhinal cortex (Witter & Amaral, 2004). Lesions of the medial prefrontal cortex in rabbits impair trace eyeblink conditioning (Kronforst-Collins & Disterhoft, 1998; McLaughlin, Skaggs, Churchwell, & Powell, 2002; Powell, Churchwell, & Burriss, 2005; Weible, McEchron, & Disterhoft, 2000). In addition, the fact that neurons in the medial prefrontal cortex respond early in trace eyeblink conditioning to the CS (Weible et al., 2003) parallels the time course of activation to the trace CS that we observed in interpositus neurons.
The interpositus nucleus, in turn, relays conditioning-related information to forebrain regions and it may be that differential interpositus nucleus activity during delay and trace conditioning is not only the result of input from forebrain regions but is itself influential in shaping that input. Lesions of the interpositus nucleus that abolish or prevent the development of the eyeblink CR also abolish or prevent the development of the hippocampal model of the CR in delay eyeblink conditioning (Clark, McCormick, Lavond, & Thompson, 1984; Sears & Steinmetz, 1990). The pathway by which the interpositus nucleus projects eyeblink CR-related information to the hippocampus is currently unknown but is assumed to be via the thalamus (Katz & Steinmetz, 2002), with the ventrolateral thalamic nucleus being ruled out as a relay from the cerebellum for at least the maintenance of previously established CR-related activity in the hippocampus (Sears, Logue, & Steinmetz, 1996). In addition, it is also unknown whether interpositus nucleus lesions completely abolish trace eyeblink conditioning-related activity in the hippocampus.
Besides the possibility that forebrain regions are responsible for the differential activation patterns of interpositus nucleus neurons that we observed during delay versus trace conditioning, it may be that cerebellar cortex plays a role. The role of cerebellar cortex in delay eyeblink conditioning has been a topic of controversy for some time, with several possible (and overlapping) functions attributed to it, including modulation of the rate of acquisition (Chen, Bao, Lockard, Kim, & Thompson, 1996; Lavond & Steinmetz, 1989), consolidation of learning (Attwell, Cooke, & Yeo, 2002; Cooke, Attwell, & Yeo, 2004), and control of CR timing (Bao, Chen, Kim, & Thompson, 2002; Medina, Garcia, Nores, Taylor, & Mauk, 2000; Perrett, Ruiz, & Mauk, 1993). Recent studies using mutant mice with either deficient long-term depression at parallel fiber-to-Purkinje cell synapses (Kishimoto, Hirono et al., 2001; Kishimoto, Kawahara, Fujimichi et al., 2001; Kishimoto, Kawahara, Suzuki et al., 2001) or abnormalities in Purkinje cell physiology (Woodruff-Pak et al., 2006) support a role for cerebellar cortex in eyeblink CR acquisition in delay but not in trace conditioning. To date, there are no published studies of Purkinje cell activity during trace eyeblink conditioning but the results with mutant mice suggest that Purkinje cell inhibition and disinhibition of the interpositus nucleus may not be closely related to the development of CRs in trace conditioning. Studies comparing Purkinje cell activity during delay and trace eyeblink conditioning may be very informative.
4.3. Summary
In summary, when delay and trace conditioning are matched for CS-US interval, the current results suggest that hippocampal CA1 pyramidal cell activity is shaped by detection of the CS, which takes longer in trace conditioning. In addition, the current results suggest that cerebellar interpositus nucleus neurons are more responsive early in trace conditioning to the first part of the CS-US interval, when the CS is present, compared to delay conditioning and are more responsive later in trace conditioning to the second part of the CS-US interval, when the CS is not present, compared to delay conditioning. The pattern of activity in the interpositus nucleus during trace conditioning may reflect the influence of forebrain structures necessary for trace conditioning to occur.
Acknowledgments
Support for this research came from a Junior Faculty Project Grant from NIH/NCRR P20 RR16435 from the National Center for Research Resources and generous startup funds from the Vermont Genetics Network through NIH/NCRR P20 RR16462 from the BRIN Program of the National Center for Research Resources. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. We would like to thank William Falls for his comments on an earlier draft of this manuscript.
References
- Akase E, Alkon DL, Disterhoft JF. Hippocampal lesions impair memory of short-delay conditioned eye blink in rabbits. Behavioral Neuroscience. 1989;103:935–943. doi: 10.1037//0735-7044.103.5.935. [DOI] [PubMed] [Google Scholar]
- Attwell PJE, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34:1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proceedings of the National Academy of Sciences. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger TW, Bassett JL. System properties of the hippocampus. In: Gormezano I, Wasserman ER, editors. Learning and Memory: The Behavioral and Biological Substrates. Erlbaum; Hillsdale, NJ: 1992. pp. 275–320. [Google Scholar]
- Berger TW, Berry SD, Thompson RF. Role of the hippocampus in classical conditioning of aversive and appetitive behaviors. In: Isaacson RL, Pribram K. h., editors. The Hippocampus. Vol. 4. Plenum Press; New York: 1986. pp. 203–239. [Google Scholar]
- Berger TW, Rinaldi PC, Weisz DJ, Thompson RF. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. Journal of Neurophysiology. 1983;50:1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- Berger TW, Thompson RF. Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response I. the hippocampus. Brain Research. 1978a;145:323–346. doi: 10.1016/0006-8993(78)90866-1. [DOI] [PubMed] [Google Scholar]
- Berger TW, Thompson RF. Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response II. septum and mammillary bodies. Brain Research. 1978b;156:293–314. doi: 10.1016/0006-8993(78)90510-3. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Activity of deep cerebellar nuclear cells during classical conditioning of nictitating membrane extension in rabbits. Experimental Brain Research. 1990;83:44–54. doi: 10.1007/BF00232192. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiology of Learning and Memory. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Collier AC, Bouton ME, Marlin NA. Some tricks for ameliorating the trace-conditioning deficit. Bulletin of the Psychonomic Society. 1978;11:403–406. [Google Scholar]
- Burman MA, Gewirtz JC. Timing of fear expression in trace and delay conditioning measured by fear-potentiated startle in rats. Learning & Memory. 2004;11:205–212. doi: 10.1101/lm.66004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Steinmetz JE. A general-purpose computer system for behavioral conditioning and neural recording experiments. Behavior Research Methods, Instruments, & Computers. 1998;30:384–391. [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JJ, Thompson RF. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. Journal of Neuroscience. 1996;16(8):2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: Acquisition and retention. Learning & Memory. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark GA, McCormick DA, Lavond DG, Thompson RF. Effects of lesions of cerebellar nuclei on conditioned behavioral and hippocampal neuronal responses. Brain Research. 1984;291:125–136. doi: 10.1016/0006-8993(84)90658-9. [DOI] [PubMed] [Google Scholar]
- Clark RE, Manns JR, Squire LR. Trace and delay eyeblink conditioning: Contrasting phenomena of declarative and nondeclarative memory. Psychological Science. 2001;12:304–308. doi: 10.1111/1467-9280.00356. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Human eyeblink classical conditioning: Effects of manipulating awareness of the stimulus contingencies. Psychological Science. 1999;10:14–18. [Google Scholar]
- Cooke SF, Attwell PJE, Yeo CH. Temporal properties of cerebellar-dependent memory consolidation. Journal of Neuroscience. 2004;24:2934–2941. doi: 10.1523/JNEUROSCI.5505-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Ranck JB. Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Experimental Brain Research. 1981;41:399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Nicholson DA. Neuronal activity in the cerebellar interpositus and lateral pontine nuclei during inhibitory classical conditioning of the eyeblink response. Brain Research. 1999;833:225–233. doi: 10.1016/s0006-8993(99)01547-4. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behavioral Neuroscience. 2005a;119:164–179. doi: 10.1037/0735-7044.119.1.164. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behavioral Neuroscience. 2005b;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Green JT, Johnson TB, Goodlett CR, Steinmetz JE. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learning and Memory. 2002;9:304–320. doi: 10.1101/lm.47602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Woodruff-Pak DS. Eyeblink classical conditioning: Hippocampal formation is for neutral stimulus associations as cerebellum is for association-response. Psychological Bulletin. 2000;126:138–158. doi: 10.1037/0033-2909.126.1.138. [DOI] [PubMed] [Google Scholar]
- Gruart A, Guillazo-Blanch G, Fernandez-Mas R, Jimenez-Diaz L, Delgado-Garcia JM. Cerebellar posterior interpositus nucleus as an enhancer of classically conditioned eyelid responses in alert cats. Journal of Neurophysiology. 2000;84:2680–2690. doi: 10.1152/jn.2000.84.5.2680. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Stanton ME. Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiology of Learning and Memory. 2001;76:426–446. doi: 10.1006/nlme.2001.4027. [DOI] [PubMed] [Google Scholar]
- James GO, Hardiman MJ, Yeo CH. Hippocampal lesions and trace conditioning in the rabbit. Behavioural Brain Research. 1987;23:109–116. doi: 10.1016/0166-4328(87)90048-9. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Hearst E. Bridging temporal gaps between CS and US in autoshaping: Insertion of other stimuli before, during, and after CS. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:187–203. [PubMed] [Google Scholar]
- Katz DB, Rogers RF, Steinmetz JE. Novel factors contributing to the expression of latent inhibition. Behavioral Neuroscience. 2002;116:824–836. doi: 10.1037//0735-7044.116.5.824. [DOI] [PubMed] [Google Scholar]
- Katz DB, Steinmetz JE. Psychological functions of the cerebellum. Behavioral and Cognitive Neuroscience Reviews. 2002;1:229–241. doi: 10.1177/1534582302001003004. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Cool V, Gormezano I. Trace conditioning of the rabbit’s nictitating membrane response as a function of CS-US interstimulus interval and trials per session. Learning and Motivation. 1991;22:269–290. [Google Scholar]
- Kehoe EJ, Napier RM. In the blink of an eye: Real-time stimulus factors in delay and trace conditioning of the rabbit’s nictitating membrane response. Quarterly Journal of Experimental Psychology. 1991;43B:257–277. [PubMed] [Google Scholar]
- Kehoe EJ, Weidemann G. Within-stimulus competition in trace conditioning of the rabbit’s nictitating membrane response. Psychobiology. 1999;27:72–84. [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 1995;109(2):195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- King DAT, Tracy J. DataMunch: A Matlab m-file collection for the analysis of trial-based spike and behavioral data. Authors; Bloomington, IN: 1999. [Google Scholar]
- Kishimoto Y, Hirono M, Sugiyama T, Kawahara S, Nakao K, Kishio M, et al. Impaired delay but normal trace eyeblink conditioning in PLCβ4 mutant mice. Neuroreport. 2001;12:2919–2922. doi: 10.1097/00001756-200109170-00033. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Fujimichi R, Mori H, Mishina M, Kirino Y. Impairment of eyeblink conditioning in GluRδ2-mutant mice depends on the temporal overlap between conditioned and unconditioned stimuli. European Journal of Neuroscience. 2001;14:1515–1521. doi: 10.1046/j.0953-816x.2001.01772.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishina M, Kirino Y. Classical eyeblink conditioning in glutamate receptor subunit δ2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. European Journal of Neuroscience. 2001;13:1249–1253. doi: 10.1046/j.0953-816x.2001.01488.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Nakazawa K, Tonegawa S, Kirino Y, Kano M. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. Journal of Neuroscience. 2006;26:1562–1570. doi: 10.1523/JNEUROSCI.4142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiology of Learning and Memory. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behavioural Brain Research. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Lee T, Kim JJ. Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. Journal of Neuroscience. 2004;24:3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Awareness predicts the magnitude of single-cue trace eyeblink conditioning. Hippocampus. 2000a;10:181–186. doi: 10.1002/(SICI)1098-1063(2000)10:2<181::AID-HIPO7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Parallel acquisition of awareness and trace eyeblink classical conditioning. Learning & Memory. 2000b;7:267–272. doi: 10.1101/lm.33400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Standard delay eyeblink conditioning is independent of awareness. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:32–37. [PubMed] [Google Scholar]
- Marchand AR, Luck D, DiScala G. Trace fear conditioning: A role for context? Archives Italiennes de Biologie. 2004;142:251–263. [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. Journal of Neuroscience. 1984;4:2811–2822. doi: 10.1523/JNEUROSCI.04-11-02811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. Journal of Neurophysiology. 1997;78:1030–1044. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9:385–396. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and Pavlovian conditioning: Trace versus delay conditioning. Behavioral Neuroscience. 2002;116:37–47. [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: Testing predictions of a large-scale computer simulation. Journal of Neuroscience. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Munera A, Gruart A, Munoz MD, Fernandez-Mas R, Delgado-Garcia JM. Hippocampal pyramidal cell activity encodes conditioned stimulus predictive value during classical conditioning in alert cats. Journal of Neurophysiology. 2001;86:2571–2582. doi: 10.1152/jn.2001.86.5.2571. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH. Neuronal correlates of conditioned inhibition of the eyeblink response in the anterior interpositus nucleus. Behavioral Neuroscience. 2002;116:22–36. [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port RL, Mikhail AA, Patterson MM. Differential effects of hippocampectomy on classically conditioned rabbit nictitating membrane response related to interstimulus interval. Behavioral Neuroscience. 1985;99:200–208. doi: 10.1037//0735-7044.99.2.200. [DOI] [PubMed] [Google Scholar]
- Port RL, Romano AG, Steinmetz JE, Mikhail AA, Patterson MM. Retention and acquisition of classical trace conditioned responses by rabbits with hippocampal lesions. Behavioral Neuroscience. 1986;100:745–752. doi: 10.1037//0735-7044.100.5.745. [DOI] [PubMed] [Google Scholar]
- Powell DA, Churchwell J, Burriss L. Medial prefrontal lesions and Pavlovian eyeblink and heart rate conditioning: Effects of partial reinforcement on delay and trace conditioning in rabbits (Oryctolagus cuniculus) Behavioral Neuroscience. 2005;119:180–189. doi: 10.1037/0735-7044.119.1.180. [DOI] [PubMed] [Google Scholar]
- Ranck JB. Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Experimental Neurology. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Levy WB. A model of hippocampal activity in trace conditioning: Where’s the trace? Behavioral Neuroscience. 2001;115:1224–1238. doi: 10.1037//0735-7044.115.6.1224. [DOI] [PubMed] [Google Scholar]
- Rogers RF, Britton GB, Steinmetz JE. Learning-related interpositus activity is conserved across species as studied during eyeblink conditioning in the rat. Brain Research. 2001;905:171–177. doi: 10.1016/s0006-8993(01)02532-x. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJH. Precerebellar nuclei and red nucleus. In: Paxinos G, editor. The Rat Nervous System. 3rd ed. Elsevier; San Diego, CA: 2004. pp. 167–204. [Google Scholar]
- Schmaltz LW, Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbit (oryctolagus cuniculus) Journal of Comparative Physiological Psychology. 1972;79:328–333. doi: 10.1037/h0032531. [DOI] [PubMed] [Google Scholar]
- Sears LL, Logue SF, Steinmetz JE. Involvement of the ventrolateral thalamic nucleus in rabbit classical eyeblink conditioning. Behavioural Brain Research. 1996;74:105–117. doi: 10.1016/0166-4328(96)00171-4. [DOI] [PubMed] [Google Scholar]
- Sears LL, Steinmetz JE. Acquisition of classically-conditioned-related activity in the hippocampus is affected by lesions of the cerebellar interpositus nucleus. Behavioral Neuroscience. 1990;104:681–692. doi: 10.1037//0735-7044.104.5.681. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Moore JW. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. Journal of Comparative and Physiological Psychology. 1975;89(10):1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- Solomon PR, VanderSchaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Toward a modern theory of adaptive networks: Expectation and prediction. Psychological Review. 1981;88:135–170. [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. Journal of Neuroscience. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Takatsuki K, Kirino Y. Time-limited role of the hippocampus in the memory for trace eyeblink conditioning in mice. Brain Research. 2002;951:183–190. doi: 10.1016/s0006-8993(02)03159-1. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14:58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behavioral Neuroscience. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Activity profiles of single neurons in caudal anterior cingulate cortex during trace eyeblink conditioning in the rabbit. Journal of Neurophysiology. 2003;90:599–612. doi: 10.1152/jn.01097.2002. [DOI] [PubMed] [Google Scholar]
- Weiss C, Knuttinen M-G, Power JM, Patel RI, O’Connor MS, Disterhoft JF. Trace eyeblink conditioning in the freely moving rat: Optimizing the conditioning parameters. Behavioral Neuroscience. 1999;113:1100–1105. doi: 10.1037//0735-7044.113.5.1100. [DOI] [PubMed] [Google Scholar]
- Weiss C, Kronforst-Collins MA, Disterhoft JF. Activity of hippocampal pyramidal neurons during trace eyeblink conditioning. Hippocampus. 1996;6:192–209. doi: 10.1002/(SICI)1098-1063(1996)6:2<192::AID-HIPO9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. 3rd ed. Elsevier; San Diego, CA: 2004. pp. 635–704. [Google Scholar]
- Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel Scn8A (Nav1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behavioral Neuroscience. 2006;120:229–240. doi: 10.1037/0735-7044.120.2.229. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: Abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Research. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]


