Abstract
The study purpose was to compare teacher ratings of academic performance (TRP) over 24 months between children with new-onset seizures (N = 121) and new-onset asthma (N = 54) ages 4 to 14 years. At each data collection point (baseline, 12 months, 24 months), children with seizures were placed into two groups according to their recurrent seizure status (yes/no) during that period. Longitudinal linear mixed models were used to explore differences between the asthma group and the two seizure groups and to identify if differences in TRP in children with seizures were associated with age, gender, or use of medication. In the seizure sample, scores for children in both groups (with and without recurrent seizures) initially declined at 12 months; however, at 24 months, children who did not have recurrent seizures improved while children who continued to have recurrent seizures declined. There was a trend for younger children to decline more than older children.
Keywords: academic achievement, pediatric, epilepsy, school, teacher ratings
Children with chronic seizures experience more academic problems than healthy children or children with other chronic health conditions, such as asthma (1–6), and also make less academic progress than would be expected for their age and intelligence quotient (IQ) level, (7, 8). In longitudinal studies, children with chronic epilepsy failed to show improvement in academic performance over time and, despite an improvement in their seizure conditions, continued to perform worse than a healthy control group (9) and an asthma comparison sample (10).
Although the majority of studies of children with chronic seizures have found academic difficulties, studies are less consistent in delineating factors predicting underperformance. Cross- sectional studies have demonstrated associations between academic performance and several demographic, neurological, and seizure-related variables, including age, gender, certain antiepileptic medications (AEDs), and seizure frequency. Younger age at onset of seizures has been associated with higher rates of academic underachievement (7, 8, 11–13). Furthermore, earlier age at onset of recurrent seizures was found to be the strongest predictor of cognitive impairment, including academic achievement, in children with complex partial seizures (13). Other studies, however, found no relationships between age at onset of seizures and neurocognitive scores (14, 15). Although some early studies reported higher academic achievement for females than for males with epilepsy (16–18), others found no main effect for gender (7, 19). In a recent study, Austin and colleagues (10) found males with high severity epilepsy to have the most risk for underachievement. Cognitive problems from AEDs might result from sedation, attention difficulties, or mood disturbances. Older AEDs, including phenobarbital and benzodiazepines, and the newer AED, topiramate, have been associated with adverse cognitive effects, but studies are limited on the effects of other newer AEDs on cognitive function (20–22). Williams et al. (19) found no differences in performance on cognitive measures between children with new-onset seizures before and after six months of treatment with AEDs compared to children with recently diagnosed diabetes mellitus. Increased seizure frequency has been negatively related to performance in some studies (7, 23–26), although other studies found that seizure variables were not strongly related to academic achievement (2, 4, 14, 27).
To more clearly define the etiology and natural history of academic difficulties, longitudinal studies beginning early in the course of the seizure disorder are needed. To date, only four studies have examined academic problems among children with new-onset seizures, and in two of the studies (4, 14) data on children with both new-onset and chronic seizures were combined and not reported separately. Berg et al. (28) enrolled children 1 month to 16 years of age into a study of new-onset epilepsy and reported academic progress at a 5-year follow-up. They found that children with remote symptomatic epilepsy or an epileptic encephalopathy were more likely to receive special education services, an indirect measure of academic performance. They did not obtain specific measures of each child’s academic performance. The final study by Oostrom and colleagues (27, 29) compared children enrolled in regular school classrooms who had experienced two or more unprovoked idiopathic or cryptogenic seizures within the past year with gender-matched classmate normal controls. They found that children with epilepsy scored significantly worse on academic skills (reading, writing, and math) than controls and, over the year following entry into the study, a decline in these skills was found. This decline, however, was in children whose parents had reported problems with skill acquisition prior to the onset of epilepsy. Follow-up at 3–4 years after diagnosis also found that learning problems prior to the diagnosis of epilepsy was the only variable predicting continuing academic difficulty. The sample was small (N = 51) and restricted to children with idiopathic or cryptogenic epilepsy, reducing the generalizability of the findings. Although the findings of these four studies are informative, research did not compare children with new-onset seizures to children with another chronic health condition, which makes it difficult to know if problems were associated with seizure onset or to onset of a chronic health condition. Moreover, these studies did not explore subgroups of children with new-onset seizures to determine which ones were most at risk for academic problems or for developing them over time.
In this study, we have tried to determine the natural history of cognitive problems associated with childhood-onset epilepsy. There would be three possible outcomes. First, if the academic difficulties of children with epilepsy are related to underlying central nervous system dysfunction, the child should have cognitive impairment at the onset of the seizures. Second, if the cognitive problems are the result of continuing seizures or an adverse effect of medication, academic difficulties should not be present at the onset of seizures but would emerge in those children with recurrent seizures or in those treated with AEDs. Third, if the academic problems of children with epilepsy are a nonspecific response to a chronic illness, their academic problems would worsen over time with recurrent seizures but would not differ from problems found in children with the chronic condition of asthma.
The purpose of this study was to address these three possibilities by determining if children with new-onset seizures differed on academic performance both at onset and over time compared to children with asthma. Our sample differed from previous samples in several aspects. Children were recruited immediately following a first-recognized seizure. Neurological status was monitored and academic performance was assessed using teacher ratings at baseline and at 12 and 24 months. Data from school-administered standardized achievement tests were used to validate the teachers’ ratings of performance. Children with recurrent seizures were compared to children with no recurrences and both groups were compared to a sample of children with a recent increase in severity of asthma.
To investigate which of the three possibilities was most likely, we formulated two specific research questions.
Are there differences in academic performance over time (baseline, 12 months, and 24 months) by age, gender, or group (asthma, recurrent seizure, no recurrent seizure[s])?
In the seizure sample, are there differences in academic performance over time (baseline, 12 months, 24 months) by age, gender, medication use, seizure type/epilepsy syndrome (primary generalized absence, primary generalized tonic/clonic or atonic/akinetic, primary partial, other), or seizure group (recurrent seizure, no recurrent seizure[s])?
Methods
Research Design
As part of a larger study examining how neurological variables interact with other child and family variables to predict child mental health disturbances and academic problems (30), a sample of 121 children with new-onset seizures and a comparison sample of 54 children with recent onset of more severe asthma was studied. The descriptive statistics for age, race, and education of primary caregiver (a measure of SES) for children with asthma versus seizures are shown in Table 1. Differences in these demographic variables between children with asthma and children with seizures were tested and all were non-significant (P > 0.1). As can be seen in Table 2, mean academic performance T-scores by group (asthma, recurrent seizure, no recurrent seizure[s]) and time (baseline, 12 months, 24 months) were in the average range and were within half a standard deviation of norms.
Table 1.
Demographic and Seizure Characteristics at Baseline
| Asthma (N=54) | Seizure (N=121) | |||
|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD |
| Age | 9.06 | 2.27 | 9.50 | 2.45 |
| Education of primary caregiver | 13.65 | 2.69 | 14.15 | 2.65 |
| Gender | N (%) | N (%) | ||
| Female | 26 (48.2%) | 63 (52.1%) | ||
| Race | ||||
| African American | 10 (18.5%) | 18 (14.9%) | ||
| Caucasian | 41 (75.9%) | 100 (82.6%) | ||
| Other | 3 (5.6%) | 3 (2.5%) | ||
| Seizure Type | ||||
| Generalized Tonic/Clonic | 40 (33.1%) | |||
| Absence | 10 (8.3%) | |||
| Elementary Partial | 10 (8.3%) | |||
| Complex Partial | 29 (24.0%) | |||
| Partial with Secondary Generalization | 17 (14.0%) | |||
| Unknown | 2 (1.6%) | |||
| Multiple Types | 13 (10.7%) | |||
Table 2.
Mean Achievement Scores over Time for Each Group
| Teacher’s Rating of Performance (TRP) T-scores | ||||
|---|---|---|---|---|
| No Recurrent Seizures | Visit (months since diagnosis) | N | Mean | SD |
| 0 | 73 | 49.38 | 9.22 | |
| 12 | 54 | 48.31 | 9.90 | |
| 24 | 69 | 49.87 | 9.18 | |
| Recurrent Seizures | ||||
| 0 | 45 | 49.29 | 9.81 | |
| 12 | 54 | 48.41 | 9.37 | |
| 24 | 22 | 46.82 | 10.44 | |
| Asthma | ||||
| 0 | 54 | 48.83 | 8.86 | |
| 12 | 45 | 49.42 | 8.56 | |
| 24 | 40 | 48.88 | 8.05 | |
Both samples were followed prospectively for 24 months. Participants were recruited from emergency rooms, outpatient pediatric clinics, private neurologists, and EEG laboratories. Prior to data collection, informed consent was obtained from parents and assent from children. The study was approved by the respective institutional review boards.
Seizure Sample
Children in the new-onset seizure group were included in the study if they met the following criteria: (a) were between 4 and 14 years of age; (b) had had a first-recognized seizure-like episode within the past 6 weeks that did not occur within one week following a head injury and/or was not because of a metabolic condition; (c) did not have a history of two or more febrile seizures; (c) had no other chronic medical condition requiring long-term care; (d) had an IQ of 70 or above (per school records, parent report of IQ, or placement of child in a regular classroom); and (e) did not have a sibling with a chronic medical condition. Children who had had an unrecognized seizure prior to the baseline were also eligible for the study. Children with new-onset seizures were separated into recurrent (N = 41) and no recurrent (N = 80) seizure groups. Children who were determined to have had a prior unrecognized seizure were placed into the recurrent seizure group at baseline. At 12 and 24 months, children were placed into the recurrent seizure(s) group if they had had at least one additional seizure in the prior year. Thus, a particular child could be in a different seizure group (recurrent or no recurrent seizure) at each time point (baseline, 12 months, 24 months) depending on whether he or she had had a seizure between the two time points.
Seizure variables (i.e., seizure type and epilepsy syndrome) were classified using International League Against Epilepsy criteria. Seizure type was established using parent and physician description of the seizure and results of the EEG. Syndrome was determined from the description of the seizure, child’s developmental history, results of the neurological examination, EEG results, and neuroimaging findings. These two variables were then combined into a seizure type/epilepsy syndrome variable with four classifications: primary generalized absence (N = 11), primary generalized tonic/clonic or atonic/akinetic (N = 39), primary partial (N = 26), and secondary partial or other (N = 45). These decisions were made by author DWD.
Along with other demographic statistics on Table l, seizure types are also shown. Generalized tonic/clonic and complex partial seizures accounted for over half of the seizures. At baseline, 57.9% of the children were taking an AED. This percentage increased to 66.1% at 12 months and decreased to 55.8% at 24 months. Carbamazepine, valproic acid, and phenytoin were the most commonly prescribed medications. The percentage of children taking carbamazepine was 44.4% at baseline and 20.0% at 24 months, valproic acid 37.0% at baseline and 21.9% at 24 months, and phenytoin 16.4% at baseline and 7.1% at 24 months.
Asthma Sample
Children with asthma were included in the study if they met the following criteria: (a) between 4 and 14 years of age; (b) their previously mild and infrequent asthma symptoms had become more severe within the past six weeks, which may have been indicated by a first hospitalization or treatment in the emergency room, first referral to a specialist for evaluation or management of the asthma, or initial placement on one or more daily medications; (c) had no other chronic medical condition requiring long-term care; (d) had an IQ of 70 or above (per school records, parent report of IQ, or placement of child in a regular classroom); and (e) had no siblings with a chronic medical condition. Children with worsening asthma were recruited instead of children with mild and intermittent asthma symptoms because the latter group typically receives short-term treatment for their asthma symptoms from a primary care physician. In this study, we sought to identify children whose asthma symptoms were worsening because it would indicate the onset of a more chronic condition.
At baseline, 78% of the parents of the children with asthma reported that their child had had active asthma symptoms of wheezing or coughing episodes in the past month (21% had had 30 or more episodes in the past month). The majority of children were being treated with the inhaled medications of albuterol (73.4%), cromolyn sodium (44.7%), corticosteroid (20.4), or nedocromil sodium (18.9%).
Instrumentation
Baseline data were collected within six weeks of a child having a first-recognized seizure or an indication that their asthma condition was worsening. Data collected during the baseline interview provided information about the child prior to the onset of the health condition. Health condition information was collected by parent report and medical records, academic performance was collected from the teachers, and standardized test results were obtained from school records.
Academic Performance
Performance was measured using T-scores from the teacher’s rating of performance (TRP). The TRP was collected on the Teacher’s Report Form of the Child Behavior Checklist (TRF) on which the teachers rate classroom performance in six academic subject areas on a scale from 1 (far below grade level) to 5 (far above grade) and ratings are summed to create a total score that reflects overall performance (31). Baseline school performance was obtained from the teacher who had the child in school during the two months prior to the onset of the health condition. Follow-up data were obtained from the child’s teacher at the time of the 12- and 24-month data collections, usually a different teacher at each time point. The TRP is normed for age and gender with a mean of 50 and standard deviation of 10 and was found to have good validity and reliability in past research (31).
To validate the TRP, we obtained standardized scores from group achievement tests that were administered in the schools. The total battery is a component of standardized tests providing information about specific academic skills and reflecting the curriculum used in the schools. Although different types of school-administered standardized tests are utilized by the various school systems, the content of these tests are similar across grades and the scores tend to be highly correlated. Additionally, because these tests are nationally normed and based on a normal distribution, direct comparison is possible (32). The total battery is normed for age and gender and similarly has a mean of 50 and standard deviation of 10. The tests include measures of basic academic skills such as reading, language, and math, as well as a total composite score (total battery) that is an overall measure of performance. Because of the limited testing schedule in schools (children do not undergo testing at every grade level), not all children had test results at all three data collections. We used total battery scores that were closest in time to each data collection.
Statistical Methods
For the first research question, which examined differences between the seizure and asthma groups in academic performance over time as measured by the teacher’s rating of performance, a longitudinal linear mixed model was used. A compound symmetry variance-covariance structure was assumed. The outcome variable was change from the baseline TRP T-score. Subject was treated as a random effect; group (asthma, recurrent seizure[s], no recurrent seizure[s]) and gender were fixed effects; and age, time, and the baseline TRP score were continuous covariates. Time was included as months since baseline visit.
All children had a baseline and at least one TRP follow-up score; 110 children had all three TRP scores; 44 children had only a baseline and 12-month score; and 21 had only a baseline and 24-month score. Within the group variable, recurrent seizure(s) was treated as a time-varying covariate. Because our sample was children with new-onset seizures, we thought it likely that a child could fully recover from having one seizure, as opposed to it having a permanent effect on the child’s academic performance. Thus, we decided to make the group a time-varying covariate and look at the effect of recent seizures on TRP as opposed to the effect of having an additional seizure at any time in the past. The three-way interaction term among gender, time, and group (asthma, recurrent seizure[s], and no recurrent seizure[s]), as well as the two-way interaction terms of gender by time, gender by group, and time by group were tested.
For the second research question, which focused only on the seizure sample, the analyses were similar to those for the first research question but included variables for treatment with any AEDs in the prior time interval (yes or no) and seizure type/epilepsy syndrome. The associations among age, gender, time (baseline, 12 months, 24 months), AED use (yes or no), seizure type/epilepsy syndrome, and recurrent seizure group (yes or no), adjusting for baseline TRP score, with change from the baseline TRP T-score, were investigated. The two-way interaction terms of gender by time, gender by group, and time by group were also included in the model. The interaction term of medication use by group was not included in the model because there were so few children who were having recurrent seizures but were not taking medication, especially at 24 months when only four children fit this category.
Because our sample size of children who had baseline plus one total battery score was limited (N = 77), descriptive statistics for the school-administered standardized achievement test total battery scores were calculated and qualitatively compared to the TRP T-scores.
Results
Are there differences in academic performance (TRP) over time (baseline, 12 months, 24 months) by age, gender, or group (asthma, recurrent seizure, no recurrent seizures[s])?
Neither the three-way interaction of gender, time and group, nor the two-way interactions of gender by time and gender by group, were statistically significant (P > 0.2) so the results presented are based on the model without these interaction terms. Although the interaction between group and time was not statistically significant (P = 0.098), it was kept in the model because it was suggestive of a possible interaction effect. As can be seen in Figure 1, the scores of children with asthma remained fairly constant over the 24-month period. The scores of both children with and without recurrent seizures decreased from baseline to 12 months. However, between 12 and 24 months the scores of children with recurrent seizures continued to decline, while the scores for children not experiencing recurrent seizures increased back to their baseline level. Neither gender (F (1,1601) = 0.00, P = 0.972), time (baseline, 12 months, 24 months, F (1,163) = 0.65, P = 0.422), group (asthma, recurrent seizure, no recurrent seizure[s], F (2,211) = 1.84, P = 0.162), nor age (F (1,159) = 3.66, P = 0.058 were significantly associated with the change in teachers’ ratings of child academic performance from baseline. Although age was not significantly associated with the change in TRP score from baseline, the trend was for younger children to decline more than older children. This was true for children in all groups. The average decline from baseline to 24 months was 1.6 points among children younger than 10 years of age. In children older than 10 years of age, the average decline from baseline to 24 months was 0.3 points.
Figure 1.
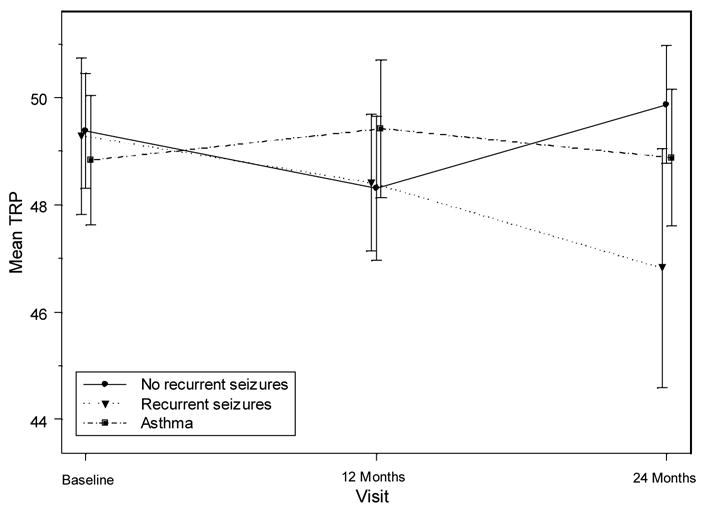
Teacher’s Rating of Academic Performance by Group
In the seizure sample, are there differences in academic performance, as measured by the TRP, over time (baseline, 12 months, 24 months) by age, gender, AED use, seizure type/epilepsy syndrome (primary generalized absence, primary generalized tonic/clonic or atonic/akinetic, primary partial, secondary partial or other), or seizure group (recurrent seizure, no recurrent seizure[s])?
There were neither significant gender-by-time nor gender-by-recurrent seizure interaction effects (P > 0.6), so the P-values are based on the model without these two interaction terms. There was a significant time-by-recurrent seizure interaction effect (P = 0.042). At 12 months, children with and without recurrent seizures declined from baseline a similar amount (0.88 and 1.07, respectively), but by 24 months this decreasing trend had reversed for children without recurrent seizures. At 24 months, the scores of children without recurrent seizures were actually 0.49 points higher than their baseline scores. In contrast, the scores of children with recurrent seizures continued to decline another 1.59 points for a total decline of 2.47 points from baseline. This is shown in Figure 1. To explain the apparent discrepancy between this analysis and the analysis for the first research question (where only a marginally significant group-by-time interaction was noted), refer to Figure 1 and note that the asthma sample means were relatively stable across time and fluctuated near the overall mean across all groups and times. This resulted in a lower estimate of the variability due to the group-by-time interaction for the model in the first research than in the second research question while the estimate of the residual variance in the two models was similar. Thus, the F-statistic for the interaction of group and time reached only marginal significance in the first research question.
There were no significant age, gender, or seizure type/epilepsy syndrome effects (F (1,106) = 3.36, P = 0.070; F (1,109) = 0.13, P = 0.719, F (3,104) = 1.56, P = 0.2047, respectively); however, there was the same age trend as when asthma children were included in the model: Younger children tended to decline more than older children. The average decline from baseline to 24 months was 2.1 points among children under 10 years of age and 0.1 points for children older than 10 years of age. There was a significant effect of medication use on academic performance (F (1,177) = 4.12, P = 0.044). Among children without recurrent seizures who were taking AEDs, scores had a slight increase of 0.42 points at 12 months but then increased 2.30 points at 24 months. Among children without recurrent seizures who were not taking AEDs, scores decreased 2.09 points from baseline to 12 months, but at 24 months, scores were 0.82 points above their 12-month score. This is shown in Figure 2. In contrast, among children with recurrent seizures who were on AEDs, scores decreased .82 points from baseline to 12 months, and decreased an additional 3.26 points at 24 months.
Figure 2.
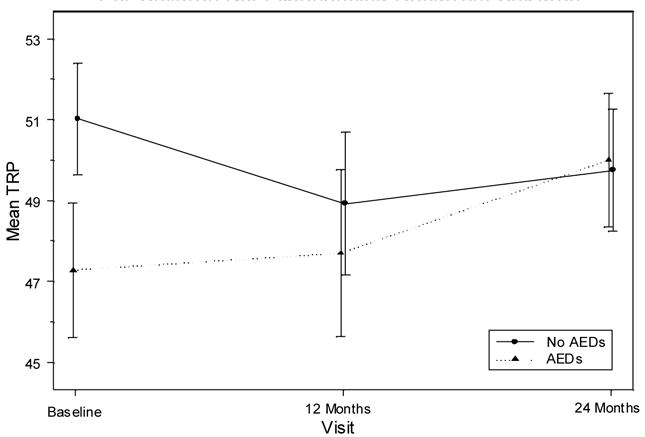
Teacher’s Rating of Academic Performance by Medication Use For Children Not Experiencing Recurrent Seizures
In a subgroup of children who had total battery scores, post-hoc analyses were conducted to validate the TRP scores. Out of the 175 children with a baseline TRP score and at least one follow-up, 26 children had three total battery scores, 51 children had a baseline battery score and one follow-up score, 23 children had only a baseline total battery score, and 43 children did not have a baseline battery score but had at least one follow-up. In general, the school-administered total battery achievement scores showed a similar pattern to the teachers’ ratings of the children’s academic performance. The means for each achievement area are shown in Tables 3 and 4. For children having recurrent seizures, both the TRP and battery scores decreased from baseline to 12 months and continued to decrease between 12 and 24 months. The total TRP score decline was 2.47 points and the total battery score decline was 9.59 points. The TRP scores in children without recurrent seizures and the battery scores in both children without recurrent seizures and children with asthma declined between baseline and 12 months and then increased between 12 and 24 months. The TRP scores for children with asthma increased between baseline and 12 months and then declined slightly between 12 and 24 months.
Table 3.
School-Administered Academic Battery Scores over Time for each Group
| Visit (Months) | N | Mean | SD | |
|---|---|---|---|---|
| No Recurrent Seizures | ||||
| 0 | 39 | 56.26 | 11.61 | |
| 12 | 31 | 54.42 | 10.97 | |
| 24 | 25 | 55.44 | 10.07 | |
| Recurrent Seizures | ||||
| 0 | 31 | 55.39 | 12.30 | |
| 12 | 36 | 55.75 | 11.12 | |
| 24 | 10 | 45.80 | 10.17 | |
| Asthma | ||||
| 0 | 29 | 56.21 | 8.50 | |
| 12 | 24 | 52.13 | 8.93 | |
| 24 | 23 | 54.35 | 8.34 |
Table 4.
School-Administered Academic Battery Subject T-scores over Time for each Group
| Visit | Reading | Language | Math | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Recurrent Seizures | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| 0 | 41 | 53.98 | 11.49 | 39 | 57.59 | 10.43 | 41 | 55.90 | 12.02 | |
| 12 | 32 | 55.13 | 11.10 | 31 | 54.35 | 10.59 | 32 | 53.91 | 11.21 | |
| 24 | 26 | 54.88 | 10.29 | 26 | 56.12 | 9.44 | 26 | 54.19 | 11.48 | |
| Recurrent Seizures | ||||||||||
| 0 | 31 | 55.35 | 11.08 | 30 | 56.30 | 12.28 | 31 | 53.65 | 11.97 | |
| 12 | 36 | 55.28 | 11.87 | 36 | 56.89 | 10.61 | 36 | 54.39 | 11.28 | |
| 24 | 10 | 45.10 | 10.35 | 11 | 49.00 | 9.48 | 12 | 46.92 | 10.27 | |
| Asthma | ||||||||||
| 0 | 29 | 54.79 | 8.55 | 29 | 55.69 | 8.73 | 29 | 57.55 | 10.09 | |
| 12 | 25 | 52.40 | 10.71 | 24 | 51.46 | 9.17 | 25 | 52.76 | 9.19 | |
| 24 | 23 | 53.70 | 8.38 | 23 | 54.65 | 9.08 | 23 | 53.22 | 9.46 | |
Discussion
The purpose of this study was to compare teacher ratings of academic performance over a 24-month period (baseline, 12 months, and 24 months) between children with new-onset seizures and asthma. Three outcome possibilities were tested. The first, that if the academic difficulties of children with epilepsy were caused by underlying central nervous system dysfunction, the child would have cognitive impairment at the onset of the seizures, was not supported. Children with seizures were not having problems at condition onset as indicated by performance scores within the normal range. The finding that children with asthma were performing well academically is consistent with the literature showing average to above average achievement (2, 10, 33–35) and few illness-related academic problems in this population (33, 36). However, finding that the children with seizures were doing well academically contrasts with prior research that found achievement problems in children with chronic seizures (1–6). Our results suggest that the achievement problems found in children with epilepsy may develop over time and might be related to the epileptic condition. At the beginning, these problems may be more subtle, but over time appear to become more pronounced. Our discovery of a decline at 24 months associated with recurrent seizures provides partial support for this interpretation.
The second possibility, that achievement problems were the result of continuing seizures or an adverse effect of medication, and therefore academic difficulties would not be present at the onset of seizures but would emerge in those children with recurrent seizures or in those on antiepileptic drugs, was supported. We found that scores for children in both groups (with and without recurrent seizures) were in the normal range at onset and initially declined at 12 months, but children who did not have recurrent seizures improved and exceeded their baseline scores at 24 months. The children who continued to have recurrent seizures showed further decline at 24 months. There was a trend for the performance scores of children who were seizure free on AEDs to improve over the 24 months. These findings are consistent with past literature supporting the relationship between active seizures and poorer academic performance. Austin and colleagues (37) found that children with active epilepsy had lower scores on school progress and intellectual self-concept than children with inactive epilepsy. Intractable epilepsy in children with onset before age two has been associated with poor cognitive functioning (38). Moreover, in the sample of children with new-onset seizures, our finding that academic performance among those with recurrent seizures tended to decline over time is consistent with results from previous research (7, 8, 10, 32), emphasizing the importance of identifying academic difficulties among children with seizures as early as possible so that interventions may be implemented that limit the progression of problems. This finding is even more important in light of the fact that our sample included only children with normal development and they were starting to show decline over time.
The last possibility, that academic problems of children with epilepsy are a nonspecific response to a chronic illness, and therefore academic problems would worsen over time with both recurrent seizures and asthma, was harder to assess. The teachers’ ratings of performance were in the average range over the 24 months for all three groups. There was no decline over the 24-month period in children with asthma nor in the children without recurrent seizures; however, there was decline in those children with recurrent seizures. This suggests that academic performance may be affected more by seizures than by the nonspecific effect of a chronic illness, but a longer period of observation in a larger sample will be required to settle this question.
Additional findings from the study were that the academic performance of younger children in the samples declined more than the older children over the two years of the study. These results are consistent with previous findings in which younger age at onset of seizures has been associated with higher rates of academic underachievement (7, 8, 11–13). Older children may have more compensatory strategies than younger children and thus are able to sustain achievement despite their chronic condition (39).
Teacher ratings of performance (TRP) were congruent with the scores obtained from the standardized achievement tests (total battery). Moreover, the pattern of change over time was also consistent between the two measures. This is important because it shows that the cognitive performance of the child in a comprehensive epilepsy clinic can be monitored using teacher reports. Whereas standardized tests are usually given every 2 to 3 years, teacher ratings occur daily, and quarterly or semester summaries can be used to closely monitor a child’s progress so that decline can be identified early, instead of waiting for the infrequent results supplied by the standardized tests.
Limitations
Excluding the population of children with mental handicap or with onset before age four who may have more severe forms of epilepsy limits the generalizability of the findings. The advantage of our sample is that it does not include many children with symptomatic or cryptogenic epilepsy, who are already known to be at significant risk for learning difficulties. Second, our sample size did not allow us to control for specific AEDs or AED blood levels. A third limitation of the study is that gross measures of academic performance were used, which did not assess the more specific components of learning that individual neurocognitive tests would provide. Although limiting, these gross measures are what is available most often for school-age children and can be useful as a way for teachers and health care professionals to monitor children’s progress at frequent intervals. The use and timing of standardized tests are also limitations of the study. A final limitation involves the uneven groups because of missing scores, with some of the seizure samples having small cell sizes, so interpretation must be tentative.
Clinical Implications
For professionals working with children with seizures, several points should be apparent. Not all children will have problems keeping up in school; however, given the high rates of academic performance problems seen in chronic samples, the initial decline seen in this new-onset sample, and the continued decline seen in the children with recurrent seizures, careful monitoring of academic performance is warranted. Additional psychoeducational assessment should be obtained when academic decline is noted. In relation to determining who may be at greatest risk for academic problems among children with new-onset seizures, findings from the current study and other research clearly suggest that recurrent seizures, as well as younger age of onset, are factors that need to be considered. Although our study was an improvement over past designs, we do not know if academic performance will continue to decline, plateau, or improve beyond two years. Further research is needed to explore the pattern of change after condition onset and to describe academic outcomes associated with living with seizures.
Acknowledgments
This research was supported by grant PHS R01 NS22416 from the National Institute of Neurological Disorders and Stroke to Joan K. Austin. We acknowledge assistance from B. Hale, B. Garg, O. Markand, as well as the Epilepsy and Pediatric Neurology Clinics at Riley Hospital, Indiana University Medical Center, Indianapolis, IN. We thank P. Dexter for editorial comments and J. Critchfield editorial assistance. Address correspondence to Dr. A.M. McNelis at Indiana University School of Nursing, 1111 Middle Dr, NU492, Indianapolis, IN 46202-5107. E-mail: ammcneli@iupui.edu
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Angela M. McNelis, Indiana University School of Nursing 1111 Middle Drive, NU 403H Indianapolis, IN 46202 Tel: (317) 274-8254 Fax: (317) 278-1811 Email: ammcneli@iupui.edu.
David W. Dunn, Indiana University School of Medicine Department of Psychiatry.
Cynthia S. Johnson, Indiana University School of Medicine Division of Biostatistics.
Joan K. Austin, Indiana University School of Nursing.
Susan M. Perkins, Indiana University School of Medicine Division of Biostatistics.
References
- 1.Feeman DJ, Hagen JW. Effects of childhood chronic illness on families. Soc Work Health Care. 1990;14:37–53. doi: 10.1300/J010v14n03_03. [DOI] [PubMed] [Google Scholar]
- 2.Huberty TJ, Austin JK, Risinger MW, McNelis AM. Relationship of selected seizure variables in children with epilepsy to performance on school-administered achievement tests. J Epilepsy. 1992a;5(1):10–6. [Google Scholar]
- 3.Huberty TJ, Austin JK, Risinger MW, McNelis AM. Classroom performance and adaptive skills in children with epilepsy. J Sch Psych. 1992b;30(4):331–42. [Google Scholar]
- 4.Mitchell W, Lee H, Chavez JM, Guzman BL. Academic underachievement in children with epilepsy. J Child Neurol. 1991;6:65–72. doi: 10.1177/088307389100600114. [DOI] [PubMed] [Google Scholar]
- 5.Rodin E, Shapiro H, Lennox K. Epilepsy and life performance. Rehabil Lit. 1977;38:34–9. [PubMed] [Google Scholar]
- 6.Rutter M, Graham P, Yule W. The prevalence of psychiatric disorder in neuro-epileptic children: A neuropsychiatric study in childhood. Clin Dev Med. 1970;35/36:175–85. [Google Scholar]
- 7.Seidenberg M, Beck N, Geisser M, Giordani B, Sackarelles JC, Berent S, et al. Academic achievement of children with epilepsy. Epilepsia. 1986;27(6):753–9. doi: 10.1111/j.1528-1157.1986.tb03606.x. [DOI] [PubMed] [Google Scholar]
- 8.Seidenberg M, Beck N, Geisser M, O’Leary DS, Giordani B, Berent S, et al. Neuropsychological correlates of academic achievement of children with epilepsy. J Epilepsy. 1988;1:23–9. [Google Scholar]
- 9.Suurmeijer TPBM. Treatment, seizure-free periods, and educational achievements: A follow-up study among children with epilepsy and healthy children. Fam Pract. 1991;8(4):320–8. doi: 10.1093/fampra/8.4.320. [DOI] [PubMed] [Google Scholar]
- 10.Austin JK, Huberty TJ, Huster GA, Dunn DW. Academic achievement in children with epilepsy or asthma. Dev Med Child Neurol. 1998;40:248–55. doi: 10.1111/j.1469-8749.1998.tb15457.x. [DOI] [PubMed] [Google Scholar]
- 11.Bourgeois BFD, Prensky AL, Palkes HS, Talent BK, Busch SG. Intelligence in epilepsy: A prospective study in children. Ann Neurol. 1983;14(4):438–44. doi: 10.1002/ana.410140407. [DOI] [PubMed] [Google Scholar]
- 12.Hermann BP, Schwartz MS, Karnes WE, Vahdat P. Psychopathology in epilepsy: Relationship of seizure type to age at onset. Epilepsia. 1980;21(1):15–23. doi: 10.1111/j.1528-1157.1980.tb04040.x. [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld J, Seidenberg M, Woodard A, Hecox K, Inglese C, Mack K, et al. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. 1999;41:724–31. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- 14.Bailet LL, Turk WR. The impact of childhood epilepsy on neurocognitive and behavioral performance: A prospective longitudinal study. Epilepsia. 2000;41(4):426–31. doi: 10.1111/j.1528-1157.2000.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 15.Sturniolo MG, Galletti F. Idiopathic epilepsy and school achievement. Arch Dis Child. 1994 May;70(5):424–8. doi: 10.1136/adc.70.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stores G. Behaviour disturbance and type of epilepsy in children attending ordinary school. New York: Raven Press; 1977. [Google Scholar]
- 17.Stores G, Hart J. Reading skills of children with generalised or focal epilepsy attending ordinary school. Dev Med Child Neurol. 1976;18:705–16. [Google Scholar]
- 18.Holdsworth L, Whitmore K. A study of children with epilepsy attending ordinary school. II: Information and attitudes held by their teachers. Dev Med Child Neurol. 1974b;16:759–65. doi: 10.1111/j.1469-8749.1974.tb03396.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams J, Bates S, Griebel ML, Lange B, Mancias P, Pihoker CM, et al. Does short-term antiepileptic drug treatment in children result in cognitive or behavioral changes? Epilepsia. 1998;39(10):1064–9. doi: 10.1111/j.1528-1157.1998.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 20.Bourgeois BFD. Differential cognitive effects of antiepileptic drugs. J Child Neurol. 2002;17:2S28–2S33. doi: 10.1177/08830738020170020901. [DOI] [PubMed] [Google Scholar]
- 21.Ortinski P, Meador KJ. Cognitive side effects of antiepileptic drugs. Epilepsy Behav. 2004;5:S60–S5. doi: 10.1016/j.yebeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Loring DW, Meador KJ. Cognitive side effects of antiepileptic drugs in children. Neurol. 2004;62:872–7. doi: 10.1212/01.wnl.0000115653.82763.07. [DOI] [PubMed] [Google Scholar]
- 23.Loiseau P, Strube E, Broustet D, Battellochi S, Gomeni C, Morselli PL. Leaning impairment in epileptic patients. Epilepsia. 1983;24:1183–92. doi: 10.1111/j.1528-1157.1983.tb04878.x. [DOI] [PubMed] [Google Scholar]
- 24.O’Leary DS, Seidenberg M, Berent S, Boll TJ. The effects of age of onset of tonic-clonic seizures on neuropsychological performance in children. Epilepsia. 1981;22:197–203. doi: 10.1111/j.1528-1157.1981.tb04102.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams J, Sharp G, Bates S, Griebel M, Lange B, Spence GT, et al. Academic achievement and behavioral ratings in children with absence and complex partial epilepsy. Educ Treat Child. 1996a;19(2):143–52. [Google Scholar]
- 26.Williams J, Sharp G, Lange B, Bates S, Griebel M, Spence GT, et al. The effects of seizure type, level of seizure control, and antiepileptic drugs on memory and attention skills in children with epilepsy. Dev Neuropsychol. 1996b;12(2):241–53. [Google Scholar]
- 27.Oostrom KJ, Smeets-Schouten A, Kruitwagen CLJJ, Peters ACB, Jennekens-Schinkel A. Not only a matter of epilepsy: Early problems of cognition and behavior in children with “epilepsy only” -- A prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112(6):1338–44. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 28.Berg AT, Smith SN, Frobish D, Levy SR, Testa FM, Beckerman B, et al. Dev Med Child Neurol. 11. Vol. 47. 2005. Nov, Special education needs of children with newly diagnosed epilepsy; pp. 749–53. [DOI] [PubMed] [Google Scholar]
- 29.Oostrom KJ, van Teeseling H, Smeets-Schouten A, Peters AC, Jennekens-Schinkel A. Three to four years after diagnosis: cognition and behaviour in children with ‘epilepsy only’. A prospective, controlled study. Brain. 2005 Jul;128(Pt 7):1546–55. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- 30.Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001 Jan;107(1):115–22. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- 31.Achenbach TM. Manual for the Teacher’s Report Form and 1991 profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991b. [Google Scholar]
- 32.Austin JK, Huberty TJ, Huster GA, Dunn DW. Does academic achievement in children with epilepsy change over time? Dev Med Child Neurol. 1999;41:473–9. [PubMed] [Google Scholar]
- 33.Gutstadt LB, Gillette JW, Mrazek DA, Fukuhara JT, LaBrecque JF, Strunk RC. Determinants of school performance in children with chronic asthma. Am J Dis Child. 1989 Apr;143(4):471–5. doi: 10.1001/archpedi.1989.02150160101020. [DOI] [PubMed] [Google Scholar]
- 34.Lindgren S, Lokshin B, Stromquist A, Weinberger M, Nassif E, McCubbin M, et al. Does asthma or treatment with theophylline limit children’s academic performance? N Engl J Med. 1992 Sep 24;327(13):926–30. doi: 10.1056/NEJM199209243271305. [DOI] [PubMed] [Google Scholar]
- 35.Milton B, Whitehead M, Holland P, Hamilton V. The social and economic consequences of childhood asthma across the lifecourse: a systematic review. Child Care Health Dev. 2004 Nov;30(6):711–28. doi: 10.1111/j.1365-2214.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 36.Annett RD, Aylward EH, Lapidus J, Bender BG, DuHamel T. Neurocognitive functioning in children with mild and moderate asthma in the childhood asthma management program. The Childhood Asthma Management Program (CAMP) Research Group. J Allergy Clin Immunol. 2000 Apr;105(4):717–24. doi: 10.1067/mai.2000.105226. [DOI] [PubMed] [Google Scholar]
- 37.Austin JK, Huster GA, Dunn DW, Risinger MW. Adolescents with active or inactive epilepsy or asthma: A comparison of quality of life. Epilepsia. 1996;37(12):1228–38. doi: 10.1111/j.1528-1157.1996.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 38.Vasconcellos E, Wyllie E, Sullivan S, Stanford L, Bulacio J, Kotagal P, et al. Mental retardation in pediatric candidates for epilepsy surgery: The role of early seizure onset. Epilepsia. 2001;42(2):268–74. doi: 10.1046/j.1528-1157.2001.12200.x. [DOI] [PubMed] [Google Scholar]
- 39.Eskritt M, Lee K. Remember where you last saw that card”: children’s production of external symbols as a memory aid. Dev Psychol. 2002 Mar;38(2):254–66. [PubMed] [Google Scholar]


