Abstract
Transforming growth factor-β (TGF-β) is a proinvasive and immunosuppressive cytokine that plays a major role in the malignant phenotype of gliomas. One novel strategy of disabling TGF-β activity in gliomas is to disrupt the signaling cascade at the level of the TGF-β receptor I (TGF-βRI) kinase, thus abrogating TGF-β–mediated invasiveness and immune suppression. SX-007, an orally active, small-molecule TGF-βRI kinase inhibitor, was evaluated for its therapeutic potential in cell culture and in an in vivo glioma model. The syngeneic, orthotopic glioma model SMA-560 was used to evaluate the efficacy of SX-007. Cells were implanted into the striatum of VM/ Dk mice. Dosing began three days after implantation and continued until the end of the study. Efficacy was established by assessing survival benefit. SX-007 dosed at 20 mg/kg p.o. once daily (q.d.) modulated TGF-β signaling in the tumor and improved the median survival. Strikingly, approximately 25% of the treated animals were disease-free at the end of the study. Increasing the dose to 40 mg/kg q.d. or 20 mg/kg twice daily did not further improve efficacy. The data suggest that SX-007 can exert a therapeutic effect by reducing TGF-β–mediated invasion and reversing immune suppression. SX-007 modulates the TGF-β signaling pathway and is associated with improved survival in this glioma model. Survival benefit is due to reduced tumor invasion and reversal of TGF-β–mediated immune suppression, allowing for rejection of the tumor. Together, these results suggest that treatment with a TGF-βRI inhibitor may be useful in the treatment of glioblastoma.
Keywords: kinases, neuroimmunology, tumor immunity
Glioblastoma (GB), the most malignant form of glioma (WHO grade IV),1 accounts for more than 21% of all primary brain and CNS tumors.2 The annual incidence of GB in the United States is 3.01 per 100,000.2 GB is an incurable cancer with a median survival of approximately 12 months from diagnosis. It has a one-year survival rate of 40%3–7 and a two-year survival rate of less than 30% despite maximal therapy.8 The current standard treatment regimen is surgical debulking (when possible), radiation therapy, and recently, the addition of cytotoxic chemotherapy using temozolomide.9 GBs are heterogeneous, infiltrative, fast-growing tumors. Although they rarely metastasize outside the CNS, complete surgical debulking is difficult. The location of the tumor and its invasive nature preclude curative resection.10 In addition, GBs are particularly malignant in that they evade cellular immune response, promote neovascularization, and damage normal brain parenchyma.
Many of the aggressive characteristics of GBs can be attributed to transforming growth factor-β (TGF-β), a cytokine that is often overexpressed in human malignant gliomas.11–18 TGF-β has been shown to promote invasiveness in glioma cell lines in vitro.19–21
Immunosuppression, which has been observed in many patients with malignant glioma,22–24 can also be attributed to TGF-β, a known potent immunosuppressive factor.25 TGF-β exerts its immunosuppressive effects by inhibiting proliferation and cytotoxic activity of brain tumor–infiltrating lymphocytes, natural killer cells, and lymphokine-activated killer cells.26,27 Elimination of glioma-derived TGF-β in animal models of glioma suppressed tumor growth.28,29 Collectively, these results suggest that the inhibition of TGF-β signaling will directly or indirectly suppress growth of malignant gliomas.
SX-007 is a small-molecule TGF-β receptor I (TGF-βRI) kinase inhibitor. To test the efficacy of this molecule in an in vivo model of GB, we chose the SMA-560 murine glioma model.30 The SMA-560 cell line was derived from a spontaneously developing astrocytic tumor in VM/Dk mice. SMA-560 cells secrete TGF-β and, when implanted orthotopically into the brain, invade into the surrounding brain tissue. Thus, this model recapitulates many of the characteristics of the human disease.
Materials and Methods
Materials and Cell Lines
SX-007 is a small-molecule inhibitor of TGF-βRI kinase (IC50 ~ 40 nM) developed by Scios Inc. (Fremont, CA, USA). SX-007 was screened against a panel of kinases as previously described and has a profile similar to that of SD-208.27
SMA-560 murine glioma cells, a kind gift of D.D. Bigner (Duke University, Durham, NC, USA), were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Cellgro, Herndon, VA, USA) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA). LN-308 human glioma cells, kindly provided by N. de Tribolet (Lausanne, Switzerland), were maintained in DMEM supplemented with 10% FCS. CCL64 mink lung epithelial cells (ATCC, Rockville, MD, USA) were maintained in DMEM supplemented with 2 mM l-glutamine (Gibco Life Technologies, Inc., Grand Island, NY, USA), 10% FCS (Biochrom), and penicillin (100 IU/ml)/streptomycin (100 μg/ml; Gibco Life Technologies, Inc.).
TGF-β Bioassay
The levels of bioactive TGF-β were determined as previously described.27 Briefly, CCL64 cells were allowed to attach to 96-well plates for 24 h and then exposed to human recombinant TGF-β1 or TGF-β2 (10 ng/ml; Peprotech, London, UK). Growth was assayed by crystal violet staining at 72 h.
Flow Cytometry
SMA-560 cells were grown to subconfluency and removed using 0.05% trypsin/0.53 mM EDTA Hank’s buffered saline solution without calcium and magnesium (Mediatech, Herndon, VA, USA). Trypsin was neutralized with DMEM/10% FCS and centrifuged for 5 min at 1,200 rpm at room temperature. Cells were counted and aliquoted to a final cell number of 1 × 106 cells/well in a 96-well round-bottom plate. Cells were washed twice with 0.1% bovine serum albumin (BSA) in Dulbecco’s phosphate-buffered saline (DPBS) and stained with 1 μg antimouse TGF-βRI/ALK-5 polyclonal antibody (R&D Systems, Minneapolis, MN, USA) for 30 min on ice. Cells were washed twice with 0.1% BSA/DPBS. Donkey antigoat IgG (heavy and light chain) fluorescein isothiocyanate (FITC; Chemicon, Temecula, CA, USA) was added at a 1/10 dilution to each well and incubated on ice for 30 min. Samples were analyzed on FACScan (BD Bioscience, San Jose, CA, USA).
Proliferation Assay
SMA-560 cells were plated in a 96-well plate. After allowing cells to attach overnight, compound was added at various concentrations. After three days, cell proliferation was measured with CellTiter 96 AQueous non-radioactive cell proliferation assay (Promega, Madison, WI, USA) according to the manufacturer’s directions.
Phosphorylated Smad ELISA
The levels of phosphorylated Smad 2/3 (p-Smad 2/3) were determined by sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well ELISA plates were coated with anti-Smad 2/3 monoclonal antibody (100 ng/well; Becton Dickinson, San Jose, CA, USA) for 18 h at 4°C. Excess antibody was removed, and the wells were treated with blocking buffer (0.3% BSA in PBS) for 2 h at room temperature. Cell lysates (125–150 μg total protein) were added to each well and incubated overnight at 4°C. Wells were rinsed before adding a polyclonal anti–p-Smad 2/3 antiserum diluted in 2% BSA/0.5% Tween-20/PBS. Following a 2 h incubation at room temperature, the wells were washed and secondary antibody was applied (horseradish peroxidase [HRP]-conjugated goat antirabbit IgG; Southern Biotech, Birmingham, AL, USA). After 1 h, the wells were developed with tetramethylbenzidine (Sigma, St. Louis, MO, USA). The plate was incubated for 5–30 min before the reaction was stopped with 0.5 N H2SO4 and read at 450 nm in a SpectraMax 250 plate reader (Molecular Devices, Sunnyvale, CA, USA). Protein concentrations were determined with a bicinchoninic acid protein assay (Pierce, Rockford, IL, USA).
Immunoblot Analysis
The levels of p-Smad 2 in glioma cells were analyzed by immunoblot using 20 μg protein per lane on 12% sodium dodecyl sulfate polyacrylamide gels. After transfer to a polyvinylidene difluoride membrane (Amersham, Piscataway, NJ, USA), the blots were blocked in PBS containing 5% skim milk and 0.05% Tween 20 and incubated overnight at 4°C with p-Smad 2 antibody (2 μg/ml; Cell Signaling Technology, Beverly, MA, USA). Visualization of protein bands was accomplished using HRP-coupled secondary antibody (Sigma, Munich, Germany) and enhanced chemiluminescence (Amersham). Total Smad 2/3 levels were assessed using a specific Smad 2/3 antibody (1 μg/ml; Becton Dickinson, Heidelberg, Germany).
In Vivo Model
The animals used in these experiments were treated in accordance with the institutional ethical guidelines of animal care, handling, and termination.
SMA-560 cells were harvested for implantation and resuspended in serum-free DMEM. A total of 5,000 cells was injected into the right striatum of each mouse on day 0. Male mice (13–15 weeks old, 30–35 g) were anesthetized by intraperitoneal injection of a cocktail of ketamine (100 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (10 mg/kg; Phoenix Pharmaceuticals Inc., St. Joseph, MO, USA). Cells were injected into the right striatum 2 mm lateral to the bregma and at a depth of 3 mm using a 5-μl Hamilton syringe that was very slowly lowered. The needle was held in place for 1 min before injecting the cells at a rate of 0.25 μl/min. After injection, the needle was kept in place for an additional 5 min before being slowly withdrawn. Once the needle was removed, the injection site was dried and the skin was sutured to close the wound. Animals were treated once with 0.1 mg/kg buprenorphine (Reckitt and Colman Pharmaceuticals Inc., Richmond, VA, USA) to alleviate postsurgical pain.
In studies where long-term survivors were rechallenged with tumor, SMA-560 cells (5,000 cells) were injected into the left side of the brain, 2 mm lateral to the bregma. Surgical procedures were similar to the procedures described above.
All animals were randomly assigned to treatment groups using GraphPad Statmate (GraphPad Prism, version 1.01i for Windows; GraphPad Software, San Diego, CA, USA) before dosing with SX-007. A suspension of SX-007 was prepared in 1% methyl cellulose in water. Dosing began three days after implanting cells and continued until the end of the study. Animals were weighed daily and dosed once a day (q.d.) by oral gavage every morning (10 ml/kg at appropriate concentration), except where noted. Animals were defined as dead if they lost > 20% of their starting weight, in accordance with institutional humane endpoint guidelines. For survival and mechanism of action studies, the end of the study was set to days 35 and 15, respectively.
Data are expressed as means ± SD. Efficacy of SX-007 was determined using Kaplan-Meier survival analysis with the log-rank posttest or two-tailed t-test (GraphPad Prism version 4.00 for Windows; GraphPad Software). Median survival was calculated at the end of the study (day 35). A p value equal to or less than 0.05 was considered statistically significant.
Pharmacokinetic Analysis
Descriptive pharmacokinetic parameters were determined by standard model-independent methods. Non-compartmental analysis was performed using WinNonlin (version 4.0.1; Pharsight, Mountain View, CA, USA). Because individual mice were not used to describe a full profile, parameters were calculated using mean data. Samples with SX-007 concentration below quantifiable limits (10 ng/ml) were assigned the value of 0 for the analysis. Nominal time points were used for all calculations. Cmax is the maximum mean plasma concentration, Tmax is the time Cmax is reached, and AUC(0–24) is the area under the plasma concentration–time curve from time 0 to 24 h for animals dosed 20 mg/kg p.o.
Smad 2/3 Phosphorylation Levels
To assess the level of p-Smad 2/3 in the brain, tissues were homogenized in 20 mM Tris-HCl, pH 7.5, containing 1 mM EDTA, 0.5% Triton X-100, 0.5% NP-40, 150 mM NaCl, 1× protease inhibitor cocktail (Roche, Palo Alto, CA, USA), and 1× phosphatase inhibitor cocktail set II (Calbiochem, San Diego, CA, USA). Tissue was homogenized using an Ultra-turrax T8 (Reyon Instruments, Brabcova, Czech Republic). Tissue homogenates were clarified by centrifugation, and the supernatant fraction was collected. The levels of p-Smad 2/3 were determined by ELISA as described above. Statistical significance was tested using a two-tailed unpaired t-test.
Real-Time Reverse Transcriptase PCR
Real-time reverse transcriptase (RT)-PCR was performed in a two-step manner. cDNA synthesis and real-time detection were carried out in a PTC-100 Thermal Cycler (MJ Research Inc., Waltham, MA, USA) and an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA), respectively. Random hexamers (Qiagen, Valencia, CA, USA) were used to generate cDNA from 200 ng RNA. TaqMan PCR Core Reagent Kit or TaqMan Universal PCR Master Mix (Applied Biosystems) was used in subsequent PCR reactions according to the manufacturer’s protocols. Relative quantitation of gene expression was performed using the relative standard curve method.
Sequence-specific primers and probes were designed using Primer Express software (version 2; Applied Biosystems). For PAI-1, the forward primer, probe, and reverse primer were 5′-ACTGCGGATGCCATCTTTG-3′, 5′-AGGGCTTCATGCCCCACTTCTTCAA-3′, and 5′-GAGAAGTCCACCTGTTTCACCAT-3′, respectively. For the 18S rRNA, the forward primer, probe, and reverse primer were 5′-GCCGCTAGAGGT-GAAATTCTTG-3′, 5′-ACCGGCGCAAGACGGACCAG-3′, and 5′-CATTCTTGGCAAATGCTTTCG-3′, respectively.
Expression levels were normalized to 18S rRNA. All real-time RT-PCR reactions were performed in triplicate.
Matrigel Invasion Assay (Boyden Chamber)
Invasion of glioma cells (104 per condition) was measured using Matrigel-coated transwell inserts (BD Biosciences, Mansfield, MA, USA).27 Briefly, transwell inserts with an 8-μm pore size were coated with Matrigel, and SMA-560 cells pretreated as indicated were applied to the upper wells and allowed to transmigrate through the membrane toward conditioned medium derived from NIH-3T3 fibroblasts in the lower wells. Migrated cells on the lower side of the membrane were fixed, stained in toluidine blue solution (Sigma-Aldrich, St. Louis, MO, USA), and counted.
Histology and Immunohistochemistry
For mechanism of action studies, glioma-bearing mice were sacrificed by cardiac puncture 15 days after tumor implantation. The mice were perfused, and the brains were postfixed overnight in 4% paraformaldehyde (American Master Tech Scientific Inc., Lodi, CA, USA); brains were then embedded in paraffin. From each brain, a 3-mm slab was cut around the SMA-560 cell injection site, and 5-μm paraffin sections were cut at 150-μm intervals. The sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin (American Master Tech), rat antimouse monoclonal CD34 IgG2a (1:100; CL8927AP, Cedarlane, Hornby, Canada), goat antimouse CD3 (1:100; SC-1127, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or rabbit antimouse active caspase 3 (1:400; AF835, R&D Systems, Minneapolis, MN, USA). Biotinylated secondary antibodies (1:150; Zymed, South San Francisco, CA, USA) were used for detection. Streptavidin alkaline phosphatase (1:100) was added, and the staining was developed with naphthol as substrate and levamisole as inhibitor of endogenous alkaline phosphatase (Fast Red Tablets, Roche). The negative control antibodies were normal rat IgG2a (Chemicon International, Temecula, CA, USA) for CD34, normal rabbit IgG for caspase 3, and normal goat IgG for CD3 (both from Santa Cruz Biotechnology).
The total number of CD34+ microvessels was counted in an area of 0.63 mm2 corresponding to two high-power fields in two nonconsecutive sections in the tumor center. To assess the degree of apoptosis, the total number of active caspase 3–positive cells was counted in the tumor center in two nonconsecutive sections. To assess the degree of T-cell infiltration, the total number of CD3+ cells was counted in the center section of the tumor. All images were analyzed using ImageProPlus (Media Cybernetics, Silver Spring, MD, USA). Tumor volume, Vt, was calculated using the formula Vt = intersectional distance × area of the section. The intersectional distance was 0.15 mm. Satellite tumors were defined as foci of glioma cells that were not connected to the main tumor. The total number of satellite tumors was determined using ImageProPlus. One of the vehicle-treated animals was excluded from the analysis because the main tumor was extremely small. Statistics were calculated using two-tailed unpaired t-tests.
CD107a Mobilization Assay
VM/Dk mice implanted intracranially with 5,000 SMA-560 cells were dosed three days postimplant with either 1% methylcellulose in water or 40 mg/kg SX-007 q.d. by oral gavage. Twelve to fifteen days postimplant (predetermined time point when animals start manifesting highest disease severity, based on data from several experiments), the animals were euthanized to collect spleen and cervical lymph nodes. Single-cell suspensions of individual spleens and pooled lymph nodes of four animals from each group were used as effectors in CD107a mobilization assay,31 with the following modifications.
Effectors (1 × 106 cells per well) were cultured in the presence or absence of SMA-560 cells (effector: target ratio of 1:10) in 96-well U-bottom tissue culture plates containing 200 μl RPMI-1640 medium (Cellgro) supplemented with 10% FCS per well. Effectors were stimulated in the presence of 5 μg/ml monensin (Sigma) and 2 μg FITC-CD107a antibody (BD Pharmingen, San Jose, CA, USA). A concentration of 500 nM SX-007 was included in the culture of lymphocytes from SX-007–treated animals throughout the culture period. Equivalent volume of dimethyl sulfoxide was used as vehicle in the culture of lymphocytes from 1% methylcellulose-treated animals. After 24 h of culture, the cells were surface stained for CD8 and CD3e (BD Pharmingen), and a minimum of 30,000 live events were collected on a BD LSRII flow cytometer (BD Bioscience, San Jose, CA). Controls included cells that were fixed and stained for determining the intracellular (total) levels of CD107a, cells stimulated with plate-bound CD3 (clone 145-2C11 in NA/LE format from BD Pharmingen, for determining antigen-independent cytolytic activity), and isotype controls. Postacquisition analysis was performed on FACS Diva software (Becton Dickinson).
Results
SX-007 Is a Potent Inhibitor of Autocrine TGF-β Signaling in Glioma Cells
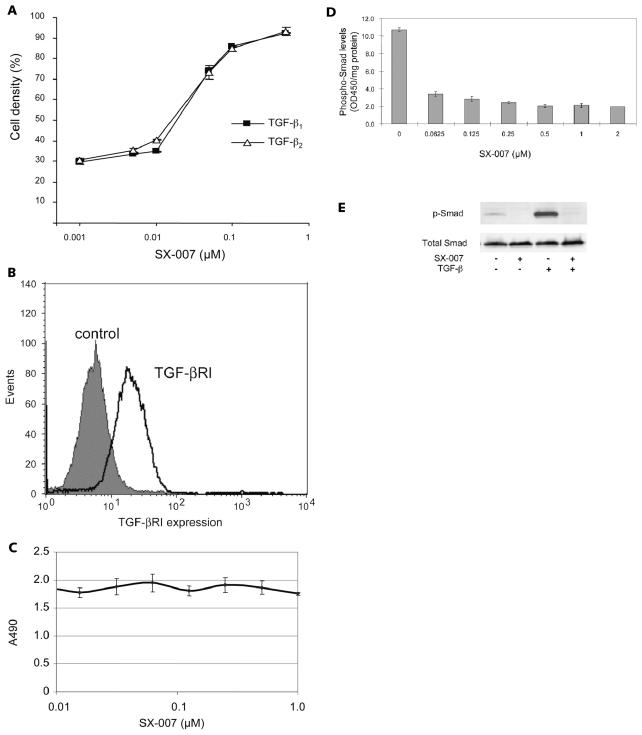
SX-007 is an inhibitor of the TGF-βRI kinase, with a half-maximal effective concentration (EC50) of approximately 0.04 μM with purified enzyme. To demonstrate the potency of this compound in a cellular system, the CCL64 bioassay was used. These cells are growth arrested when treated with TGF-β. To test the potency of SX-007, CCL64 cells were treated with either TGF-β1 or TGF-β2 (Fig. 1A). With increasing concentrations of SX-007, the TGF-β–mediated growth suppression was relieved at an EC50 of approximately 0.05 μM. SX-007 equally inhibited both TGF-β 1– and TGF-β 2–stimulated suppression of proliferation. The effects of SX-007 were also tested on SMA-560 glioma cells directly. These cells express the target, TGF-βRI (Fig. 1B). Concentrations of
Fig. 1.
SX-007 inhibits TGF-β signaling in cells. (A) TGF-β–sensitive CCL64 cells were treated with recombinant TGF-β 1 or TGF-β 2 (10 ng/ml) for 72 h in the absence or presence of SX-007. Cell number in the absence of TGF-β and SX-007 was defined as 100% (mean ± SD, n =3). (B) SMA-560 cells express the target, TGF-βRI. SMA-560 cells were stained with a polyclonal antiserum against TGF-βRI. Cells were analyzed by flow cytometry. Control = secondary antibody only. (C) SX-007 does not affect proliferation of SMA-560 cells. SMA-560 cells were treated with indicated concentrations of SX-007 for three days. Proliferation was assessed by measuring MTS formazan production. (D) SMA-560 cells were starved for 2 h in serum-free medium. Smad phosphorylation was induced with 10% serum in the presence or absence of SX-007. Smad phosphorylation was measured by enzyme-linked immunosorbent assay. (E) Protein lysates from untreated cells or cells preexposed to SX-007 (0.3 μM) for 24 h and then exposed (or not exposed) to TGF-β 2 (5 ng/ml) for 1 h in the further absence or presence of SX-007 were assessed for the levels of p-Smad 2 or total Smad 2/3. Note that the antibodies are specific for p-Smad 2 but not for total Smad 2 and 3.
SX-007 of up to 1 μM had no effect on the proliferation of SMA-560 cells (Fig. 1C). This is consistent with other TGF-βRI inhibitors, which showed no effect on proliferation in vivo or in vitro.27 SX-007 also inhibits TGF-β signaling in SMA-560 cells. Increasing concentrations of SX-007 inhibit the phosphorylation of Smad 2/3 with an IC50 of approximately 0.05 μM, in agreement with the EC50 values for the enzyme and CCL64 bioassay (Fig. 1D). Neither exogenously added TGF-β nor inhibition of TGF-β signaling with SX-007 affected total cellular levels of Smad 2/3 in SMA-560 cells (Fig. 1E).
SX-007 Improves Survival in SMA-560 Murine Glioma Model
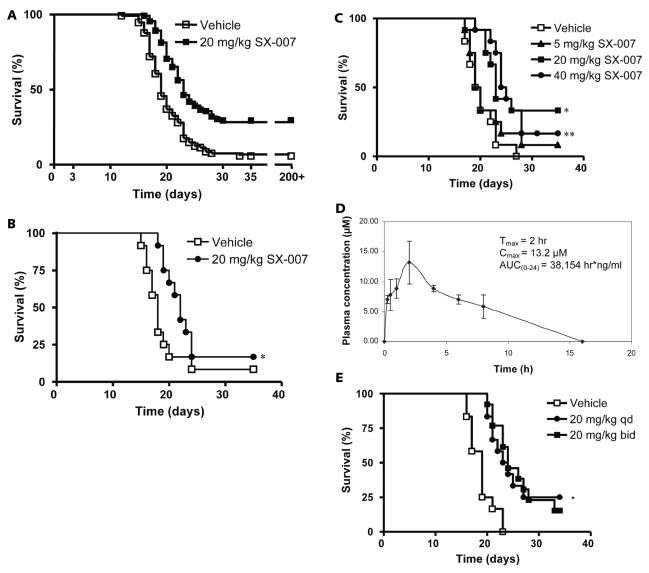
The SMA-560 murine glioma model was chosen to evaluate the therapeutic activity of SX-007. This glioma model was chosen because it recapitulates many characteristics of the human disease. In addition, it is a syngeneic model employing an immunocompetent host. The tumor is highly invasive and secretes TGF-β, as do human gliomas.11–14,16–18 The tumors were allowed to establish for three days before q.d. dosing by oral gavage (vehicle or 20 mg/kg SX-007 p.o. q.d.) until the end of the study (day 35). Survival benefit was determined using Kaplan-Meier analysis. In a total of 12 studies, there was a modest median survival benefit for SX-007–treated animals, ranging from –0.5 to 4.5 days. Fig. 2A represents a summary of the pooled data. Increased median survival was also observed in animals when dosing was initiated as late as day 11 (Fig. 2B). In addition, long-term survivors were observed in all of the studies: 7 (6% of total animals) in the vehicle groups and 33 (30% of total animals) in the SX-007–treated groups. These animals remained alive for >200 days even when SX-007 treatment was discontinued after day 35 of the study. Out of 33 long-term survivors from the SX-007–treated group, two animals died, presumably due to regrowth of tumor. The rest of the long-term survivors (31, 28% of total animals) remained disease-free.
Fig. 2.
SX-007 prolongs survival in SMA-560 model of glioma. (A) Summary graph of efficacy studies. SMA-560 cells were implanted into the right striatum of VM/Dk mice. On day 3, animals were dosed with vehicle or 20 mg/kg SX-007. Although dosing ended on day 35, animals survived past day 200. The results of 12 studies were pooled together and evaluated by Kaplan-Meier analysis. Median survival after pooling data in the vehicle (n = 114) and dosed (n = 112) groups, was 19 and 23 days, respectively. The increase in median survival between vehicle and treated animals ranged from −0.5 to 4.5 days. The percentage of long-term survivors of treated animals ranged from 0 to 60%. For the vehicle-treated animals, the percentage of long-term survivors ranged from 0 to 25%. (B) SX-007 is efficacious in late-stage disease. Animals (n = 12 for each arm) were dosed with vehicle or 20 mg/kg SX-007 p.o. once daily (q.d.) beginning day 11. Median survival of vehicle- and SX-007–treated animals was 18 and 22 days, respectively (p < 0.05, log-rank posttest). (C) Dose response with SX-007. Animals were dosed as above with vehicle or 5, 20, or 40 mg/kg p.o. q.d. Median survival in vehicle, 5 mg/kg, 20 mg/kg (*p = 0.008), and 40 mg/kg (**p = 0.009, log-rank posttest) groups was 19.5, 19.5, 23, and 24.5 days, respectively (n = 12 for each group). (D) Pharmacokinetics of SX-007 in VM/Dk mice dosed with 20 mg/kg SX-007 p.o. (E) Prolonged coverage with SX-007 does not improve efficacy. Animals were dosed as above with either vehicle (p.o. twice daily [b.i.d.]) or 20 mg/kg SX-007 (p.o. q.d. and p.o. b.i.d.; n = 12 for each group). There was no difference between the q.d. and b.i.d. groups.
SX-007 was also evaluated in a dose–response study. No significant effect on median survival was seen in the 5 mg/kg group. However, there was a significant improvement in the median survival in the 20 and 40 mg/kg groups compared with the vehicle group (median survival, 23 and 24.5 days vs. 19.5 days, p < 0.01; Fig. 2C). There was no significant difference in median survival between the 20 and 40 mg/kg groups.
Extended Inhibition of TGF-β Signaling with SX-007 Does Not Improve Efficacy
Peak plasma concentrations (14 μM) were observed 2–4 h following oral administration of SX-007 in VM/Dk mice. SX-007 is cleared to undetectable levels after 16 h (Fig. 2D). To determine whether sustaining compound levels over a longer period of time would improve efficacy, animals were dosed with SX-007 at 20 mg/kg either q.d. or twice daily by oral gavage. Surprisingly, the median survival time between the two treatment arms of this study did not differ significantly (23.5 days vs. 24 days; Fig. 2E). These results suggest that maximal effect is achieved with less than 24 h of target inhibition.
SX-007 Inhibits TGF-β Signaling in Tumor and Surrounding Brain Tissue
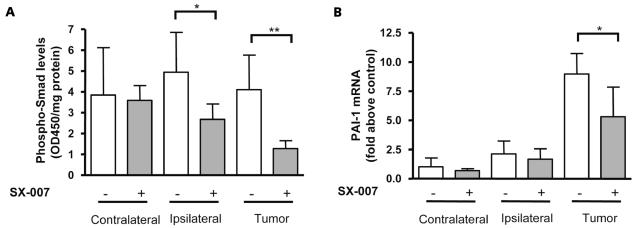
The brains of animals treated with SX-007 were collected for analysis to verify that the agent mediates TGF-β inhibition in vivo. Animals were implanted with tumor and dosed daily beginning on day 3 with 20 mg/kg SX-007 or vehicle. On day 15 of the study (12 days of dosing), the animals were given SX-007 or vehicle. Two hours after dosing, the animals were sacrificed, and plasma and brain tissue were collected. At 2 h postdose, the plasma levels were typically around 10 μM. The 2 h plasma concentration was relatively constant even after 32 days of dosing, suggesting a lack of compound accumulation (data not shown). To assess the effects of TGF-β inhibition, the levels of p-Smad 2/3, a direct downstream target of TGF-βRI kinase, were analyzed. The brain was separated into the two hemispheres. The brain tissue from the hemisphere ipsilateral to the implanted tumor was also separated from the main body of the tumor. There was 69% inhibition of p-Smad 2/3 (p < 0.01) in tumor tissue and 46% inhibition in brain tissue immediately surrounding the tumor (p < 0.05; Fig. 3A). The more pronounced effect on the ipsilateral side may be due to increased compound penetrance into the tumor. The increased penetrance may be a result of the disrupted blood–brain barrier. SX-007 can also reduce Smad 2/3 phosphorylation in the brains of healthy mice (data not shown).
Fig. 3.
SX-007 inhibits TGF-β signaling in the brain. Animals were treated with 20 mg/kg SX-007 p.o. once daily as described in Fig. 2. On day 15, the animals were sacrificed 2 h postdose, and the brain was divided into contralateral and ipsilateral hemispheres. The bulk of the tumor was also separated from the ipsilateral hemisphere. Five brains were analyzed. (A) Smad phosphorylation analysis. *p < 0.05, **p < 0.01 (two-tailed unpaired t-test). (B) TaqMan analysis of PAI-1 transcription. *p < 0.05 (two-tailed unpaired t-test).
To verify that TGF-β signaling was inhibited, the transcription levels of PAI-1, a TGF-β–mediated transcriptional event, were assayed.32 There was a 40% reduction in PAI-1 message in the tumor of SX-007–treated animals (Fig. 3B). There was no significant effect on PAI-1 transcription in the ipsilateral brain tissue (Fig. 3B). This difference between PAI-1 transcription and Smad 2/3 phosphorylation may be inherent to the tumor cells—the tumor cells appear to make more PAI-1 transcript than the surrounding tissue. These results demonstrate that SX-007 reaches tumor tissue and the surrounding tissue and that SX-007 modulates TGF-β signaling.
SX-007 Inhibits Cell Invasion
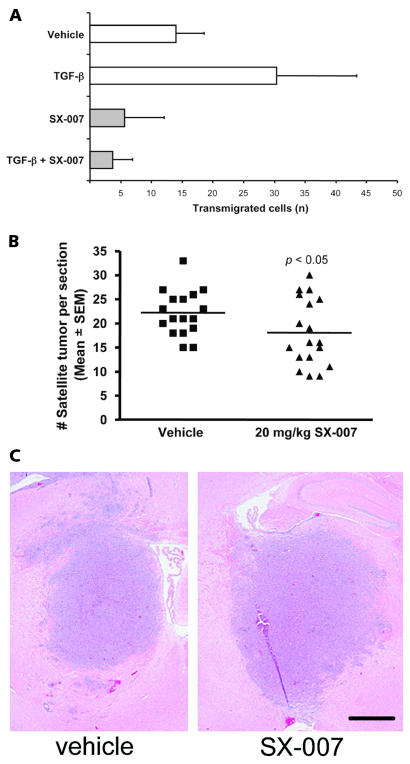
The effects of SX-007 on cell invasion were examined using a Boyden chamber migration assay. TGF-β 2 increased the invasiveness of SMA-560 cells twofold above the basal level (Fig. 4A). The invasiveness of the cells was inhibited by the addition of 0.3 μM SX-007 in the presence or absence of exogenously added TGF-β 2.
Fig. 4.
SX-007 inhibits SMA-560 motility. (A) Invasiveness was analyzed with Matrigel-coated membranes in a Boyden chemotaxis chamber assay using 104 SMA-560 cells, untreated or treated with SX-007 (0.3 μM), TGF-β 2 (5 ng/ml), or both for 24 h prior to and during the experiment, in the upper chamber. Invasive cells were counted after 24 h (mean ± SD, n = 3). (B) The number of satellite tumors (tumor foci not connected to main body of tumor) was counted in sections from animals sacrificed at day 15 of the study (n = 17 for vehicle, n = 18 for SX-007; p < 0.05, two-tailed unpaired t-test). (C) A representative micrograph showing reduced satellite tumors in SX-007–treated animals. Scale bar = 1 mm.
This in vitro result suggests that TGF-β inhibition may also reduce tumor invasiveness in vivo. To measure invasiveness in vivo, the number of satellite tumors was determined as a semiquantitative measure of tumor invasion in vivo. Satellite tumors, represented by the number of tumor foci not connected with the main tumor, were counted on day 15. These tumor foci represent tumor cells that have migrated away from the main tumor mass or are projections from the main tumor. SX-007 reduced the number of satellite tumors (22 vs. 18 satellite tumors per section, p < 0.05; Fig. 4B), suggesting that TGF-β inhibition by SX-007 has an effect on cell invasion both in vitro and in vivo. A representative micrograph is shown in Fig. 4C.
SX-007 Restores Immune Function
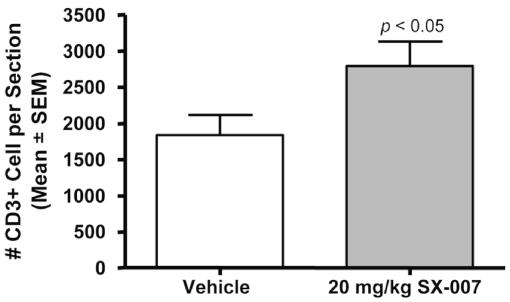
To assess the mechanistic consequences of TGF-β inhibition, the brains from tumor-bearing animals treated with vehicle or 20 mg/kg SX-007 q.d. were collected on day 15 and analyzed by histology. At this time point, there was no effect on microvessels (number, density, or size) as measured by CD34 immunohistochemistry or on proliferation as measured by Ki-67 staining or tumor size (data not shown). There was a trend of increased apoptosis in the tumors from treated animals (p = 0.09, data not shown). However, there was a statistically significant increase in CD3+ T-cell infiltration into the tumor (p < 0.05; Fig. 5). These observations along with the observed effects on cell invasion suggest that SX-007 improves survival in this model by reducing tumor infiltration and reversing the immune suppression caused by TGF-β secreted by the tumor.
Fig. 5.
SX-007 increases infiltrating CD3+ cells. The number of CD3+ cells was counted in sections from animals sacrificed at day 15 of the study (n = 17 for vehicle; n = 18 for SX-007) (p < 0.05, two-tailed unpaired t-test).
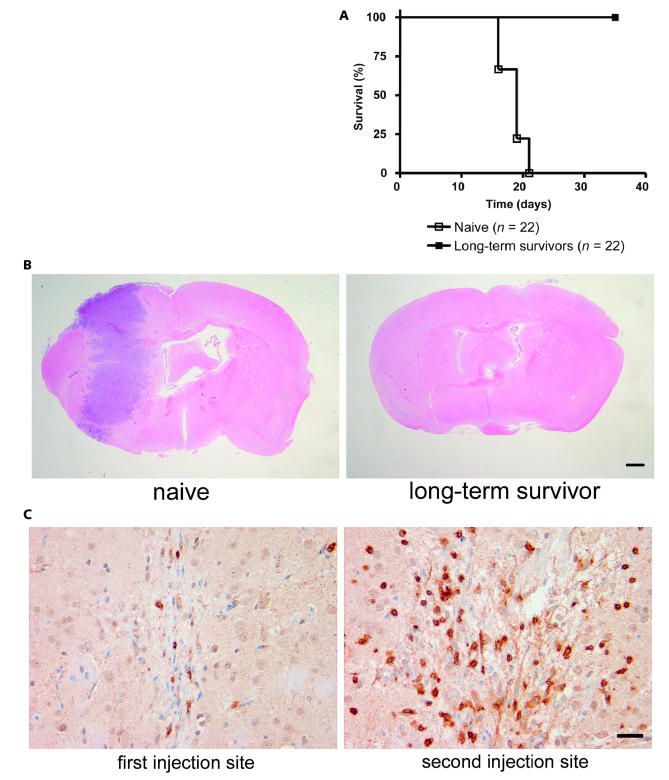
The existence of long-term survivors from the group of treated animals also suggests that immune modulation contributes to the efficacy of SX-007. To demonstrate whether these animals survived due to immune rejection of the implanted tumor, animals were rechallenged with 5,000 or 10,000 tumor cells in the contralateral side of the brain (n = 7 and 6, respectively). All rechallenged animals survived to the end of the study (Fig. 6A). During this period, the animals did not receive additional SX-007. A second study using animals rechallenged with tumor was also performed to examine histological endpoints. At day 15, the brains were collected and examined for tumor burden. There were no observable tumor cells either from the original injection or from the second injection of tumor cells in the long-term survivors (Fig. 6B). Slight scarring was seen at both implantation sites (Fig. 6C). Most important, a high concentration of CD3+ T-cells was seen in the vicinity of the second implantation site, a finding that suggests that the second tumor was rejected by mobilization of a memory T-cell response (Fig. 6C).
Fig. 6.
Long-term survivors reject tumor rechallenge. (A) Kaplan-Meier analysis of long-term survivors rechallenged with SMA-560 cells (n = 22 per group). (B) Reinjected tumor is rejected in long-term survivors. Naive animals or long-term survivors were rechallenged with SMA-560 cells in the contralateral hemisphere. Animals were sacrificed 15 days postimplantation, and the brains were harvested for histological analysis. Representative images are shown. Scale bar = 1 mm. (C) High concentration of CD3+ cells in the vicinity of the rechallenge site. Representative images are shown. Scale bar = 20 μm.
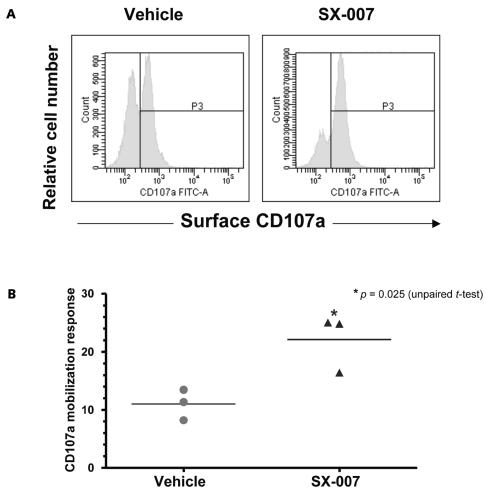
We have previously shown that in vivo treatment with a different TGF-βRI kinase inhibitor significantly increased the tumor-specific cytotoxic T-lymphocyte (CTL) activity in a mammary carcinoma model.33 Here, we used a flow cytometric assay for CD107a degranulation31 to quantify the SMA-560–specific CTL activity and to test whether in vivo inhibition of TGF-β signaling enhances tumor-specific cytolytic function. CD8+ T-cells from the cervical lymph nodes of SX-007–treated mice showed a significant (approximately twofold) increase in SMA-560–specific surface mobilization of CD107a compared to that from vehicle-treated mice (Fig. 7). Total cellular levels of CD107a did not vary significantly between the two groups (data not shown), suggesting that SX-007 treatment qualitatively enhances the tumor-specific cytolytic function of CD8+ T-cells in the draining lymph nodes.
Fig. 7.
SX-007 increases SMA-560–specific CD8+ T-cell degranulation. (A) Representative histograms of surface CD107a staining on CD8+ T-cells (CD8α+CD3ɛ+) from lymph nodes. (B) Scatter plots of percentage of tumor-specific CD8+ T-cells that mobilize CD107a to the surface. The percentage of cells that spontaneously mobilize CD107a (in the absence of SMA-560 cells) was subtracted to calculate tumor-specific activity.
Discussion
GB is a devastating disease that, despite decades of research, still remains as a major unmet medical need. The current standard of care includes radiation therapy and temozolomide treatment in addition to surgery.9 Both of these treatments are designed to be directly cyto-toxic to tumor cells. Molecularly targeted approaches such as epidermal growth factor receptor kinase inhibitors have also been tested in GB with limited success. Once again, these approaches are designed to kill the tumor directly.
Inhibition of TGF-β has gained momentum in recent years as an attractive molecularly targeted therapy. Overexpression of and/or defects in TGF-β signaling have been linked to many cancers, including lung, pancreatic, colon, prostate, and breast cancer (reviewed in Elliott and Blobe34). Current strategies to inhibit TGF-β signaling include TGF-β antisense and proteins such as antibodies or soluble TGF-β receptors.35 Both strategies are designed to block TGF-β signaling by reducing circulating TGF-β. Although these approaches may have benefit for some solid tumors, their utility is lessened in the case of GB due to the issues of drug delivery across the blood–brain barrier.
Here we describe an orally active, small-molecule inhibitor of TGF-β signaling, SX-007. The compound penetrates the brain and improves the median survival time of animals in this syngeneic, orthotopic model. In addition, it is striking that a subpopulation of tumor-bearing mice remained alive long after cessation of SX-007 treatment, suggesting that they had been cured of the tumor. Histological analyses demonstrate that immune modulation may be a mechanism by which SX-007 prolongs the survival of diseased animals. When SMA-560 cells are implanted into the brains of immunocompromised mice, SX-007 fails to prolong survival, supporting the importance of the immune system in reducing tumor burden (data not shown). The ability of long-term survivors to reject rechallenged tumor in the brain further suggests that an immune response was mounted against the first tumor.
CD107a (LAMP-1) is an integral membrane protein in cytolytic granules, and the surface mobilization of this marker on effector cells upon interaction with tumor cells has been well accepted as a measure of tumor-specific cytolytic activity.36,37 Although we could not detect cytokine-secreting function of the effectors in our assay format, we could easily detect surface mobilization of CD107a when lymph node cells were cocultured with SMA-560 tumor cells. The twofold difference in the number of CD8+ T-cells from vehicle-treated and SX-007–treated mice responding to SMA-560 cells by CD107a degranulation correlates with our similar finding that anti-mammary tumor CTL activity33 was enhanced by treating tumor-bearing mice with TGF- βRI kinase inhibitors. In addition to an increase in anti-glioma natural killer cell activity, we also reported that splenocytes from TGF-βRI kinase inhibitor–treated mice have an increased capacity to secrete interferon-γ.27 Therefore, it could be inferred that antitumor cytolytic activity is enhanced by the in vivo inhibition of TGF-β signaling and that this augmented cytolytic activity might be one of the contributing factors to the increased survival of SX-007–treated mice.
The importance of the immune response and the lack of direct tumor cell killing by SX-007 suggest that inhibition of TGF-β signaling “indirectly” clears the tumor. The observed immune effects may become maximally engaged during the period when the compound is present. Effector cell activity may continue past the point where compound is cleared from circulation. This prolonged activity may help explain why continuous inhibition of TGF-β signaling is not required for maximal efficacy. TGF-β has long been known to stimulate the motility of cells.38 We demonstrate here that SX-007 reduces the motility of SMA-560 cells both in vitro and in vivo. This direct inhibition of TGF-β signaling in tumor cells may also play a role in the increased survival of animals. It is this two-pronged approach of inhibiting signaling in both the tumor and surrounding microenvironment that distinguishes our molecule from other molecularly targeted approaches for treating GB.
Results from these in vivo investigations suggest two main modes of action: inhibition of tumor infiltration and reversal of the immune suppression allowing for tumor rejection by an immune surveillance mechanism. Both of these mechanisms make inhibition of TGF-β signaling an ideal candidate for use with other treatment modalities. For instance, temozolomide has cytotoxic activity against GB tumors but does not appear to appreciably affect the immune system. A combination of direct tumor killing with a cytotoxic agent and elimination of tumor by the host immune system may be a powerful combination therapy. Inhibition of TGF-β signaling may also be a useful cotherapy with the experimental cancer vaccines that are currently being evaluated. The results presented in these studies support the evaluation of TGF-β signaling inhibitors in patients with GB either alone or in combination with other treatment modalities.
References
- 1.Kleihues P, Cavenee WK. Pathology and Genetics of Tumours of the Central Nervous System (World Health Organization Classification of Tumours) Lyon, France: International Agency for Research on Cancer; 2000. [Google Scholar]
- 2.CBTRUS. Statistical Report: Primary Brain Tumors in the US, 1997–2001. Chicago, IL: Central Brain Tumor Registry of the United States; 2004. [Google Scholar]
- 3.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 5.Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG. Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro-Oncology. 2001;3:141–151. doi: 10.1093/neuonc/3.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 8.Van Den Bent MJ, Stupp R, Brandes AA, Lacombe D. Current and future trials of the EORTC brain tumor group. Onkologie. 2004;27:246–250. doi: 10.1159/000077974. [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 10.van den Boom J, Wolter M, Kuick R, et al. Characterization of gene expression profiles associated with glioma progression using oligonucleotide-based microarray analysis and real-time reverse transcription-polymerase chain reaction. Am J Pathol. 2003;163:1033–1043. doi: 10.1016/S0002-9440(10)63463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodmer S, Strommer K, Frei K, et al. Immunosuppression and transforming growth factor-beta in glioblastoma: preferential production of transforming growth factor-beta 2. J Immunol. 1989;143:3222–3229. [PubMed] [Google Scholar]
- 12.Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992;148:1404–1410. [PubMed] [Google Scholar]
- 13.de Martin R, Haendler B, Hofer-Warbinek R, et al. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987;6:3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjellman C, Olofsson SP, Hansson O, et al. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer. 2000;89:251–258. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Leitlein J, Aulwurm S, Waltereit R, et al. Processing of immunosuppressive pro-TGF-beta 1,2 by human glioblastoma cells involves cytoplasmic and secreted furin-like proteases. J Immunol. 2001;166:7238–7243. doi: 10.4049/jimmunol.166.12.7238. [DOI] [PubMed] [Google Scholar]
- 16.Olofsson A, Miyazono K, Kanzaki T, Colosetti P, Engstrom U, Heldin CH. Transforming growth factor-beta 1, -beta 2, and -beta 3 secreted by a human glioblastoma cell line: identification of small and different forms of large latent complexes. J Biol Chem. 1992;267:19482–19488. [PubMed] [Google Scholar]
- 17.Sasaki A, Naganuma H, Satoh E, et al. Secretion of transforming growth factor-beta 1 and -beta 2 by malignant glioma cells. Neurol Med Chir (Tokyo) 1995;35:423–430. doi: 10.2176/nmc.35.423. [DOI] [PubMed] [Google Scholar]
- 18.Stiles JD, Ostrow PT, Balos LL, et al. Correlation of endothelin-1 and transforming growth factor beta 1 with malignancy and vascularity in human gliomas. J Neuropathol Exp Neurol. 1997;56:435–439. doi: 10.1097/00005072-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Merzak A, McCrea S, Koocheckpour S, Pilkington GJ. Control of human glioma cell growth, migration and invasion in vitro by transforming growth factor beta 1. Br J Cancer. 1994;70:199–203. doi: 10.1038/bjc.1994.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulus W, Baur I, Huettner C, et al. Effects of transforming growth factor-beta 1 on collagen synthesis, integrin expression, adhesion and invasion of glioma cells. J Neuropathol Exp Neurol. 1995;54:236–244. doi: 10.1097/00005072-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Wick W, Grimmel C, Wild-Bode C, Platten M, Arpin M, Weller M. Ezrin-dependent promotion of glioma cell clonogenicity, motility, and invasion mediated by BCL-2 and transforming growth factor-beta2. J Neurosci. 2001;21:3360–3368. doi: 10.1523/JNEUROSCI.21-10-03360.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors: characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136:1631–1647. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks WH, Roszman TL, Mahaley MS, Woosley RE. Immunobiology of primary intracranial tumours. II. Analysis of lymphocyte subpopulations in patients with primary brain tumours. Clin Exp Immunol. 1977;29:61–66. [PMC free article] [PubMed] [Google Scholar]
- 24.Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–232. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 25.Weller M, Fontana A. The failure of current immunotherapy for malignant glioma: tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res Brain Res Rev. 1995;21:128–151. doi: 10.1016/0165-0173(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 26.Friese MA, Wischhusen J, Wick W, et al. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 27.Uhl M, Aulwurm S, Wischhusen J, et al. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 28.Engel S, Isenmann S, Stander M, Rieger J, Bahr M, Weller M. Inhibition of experimental rat glioma growth by decorin gene transfer is associated with decreased microglial infiltration. J Neuroimmunol. 1999;99:13–18. doi: 10.1016/s0165-5728(99)00062-4. [DOI] [PubMed] [Google Scholar]
- 29.Ständer M, Naumann U, Dumitrescu L, et al. Decorin gene transfer-mediated suppression of TGF-beta synthesis abrogates experimental malignant glioma growth in vivo. Gene Ther. 1998;5:1187–1194. doi: 10.1038/sj.gt.3300709. [DOI] [PubMed] [Google Scholar]
- 30.Serano RD, Pegram CN, Bigner DD. Tumorigenic cell culture lines from a spontaneous VM/Dk murine astrocytoma (SMA) Acta Neuropathol (Berl) 1980;51:53–64. doi: 10.1007/BF00688850. [DOI] [PubMed] [Google Scholar]
- 31.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 32.Keski-Oja J, Raghow R, Sawdey M, et al. Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin, and type I procollagen by transforming growth factor-beta: divergent responses in lung fibroblasts and carcinoma cells. J Biol Chem. 1988;263:3111–3115. [PubMed] [Google Scholar]
- 33.Ge R, Rajeev V, Ray P, et al. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Cancer Res. 2006;12:4315–4330. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- 34.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 35.Arteaga CL. Inhibition of TGFbeta signaling in cancer therapy. Curr Opin Genet Dev. 2006;16:30–37. doi: 10.1016/j.gde.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Evaluation of the CD107 cytotoxicity assay for the detection of cytolytic CD8+ cells recognizing HER2/neu vaccine peptides. Breast Cancer Res Treat. 2005;92:85–93. doi: 10.1007/s10549-005-0988-1. [DOI] [PubMed] [Google Scholar]
- 37.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 38.Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–1252. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]