Abstract
Thrombocytosis, which is defined as a platelet count greater than 400 platelets/nl, has been found to be an independent predictor of shorter survival in various tumors. Release of growth factors from tumors has been proposed to increase platelet counts. Preoperative platelet counts and other clinical and hematological parameters were reviewed from the records of 153 patients diagnosed between 1999 and 2004 with histologically confirmed glioblastoma in order to evaluate the prognostic significance of preoperative thrombocytosis in these patients. The relationship between thrombocytosis and survival was initially analyzed in all patients regardless of further therapy. Univariate log-rank tests showed that the median survival time of 29 patients with preoperative thrombocytosis (19%) was significantly shorter (4 months; 95% confidence interval [95% CI], 3–6 months) compared to 124 patients with normal platelet counts (11 months; 95% CI, 8–13 months; p = 0.0006). Multivariate analysis (Cox proportional hazards model) confirmed preoperative platelet count, age, prothrombin time, and activated partial thromboplastin time to be prognostic factors of survival (all p < 0.05). In a subset of patients (only operated patients with radiation therapy with or without additional chemotherapy), survival was likewise significantly shorter when preoperative thrombocytosis was diagnosed (6 months; 95% CI, 4–12 months) compared to patients with normal platelet count (13 months; 95% CI, 11–15 months; p = 0.0359). In multivariate analysis, age, platelet count, preoperative prothrombin time, and degree of tumor resection retained significance as prognostic factors of survival (all p < 0.05). The results of our study demonstrate preoperative thrombocytosis to be a prognostic factor associated with shorter survival time in patients with glioblastoma.
Keywords: angiogenesis, malignant glioma, platelets, prognostic parameter
Thrombocytosis, which is defined as a platelet count greater than 400 platelets/nl, has been found to be associated with significantly shorter survival times in malignant mesothelioma1; in lung,2–5 renal,6,7 colorectal,4,8 and esophageal cancer9; and in different gynecological malignancies and breast cancer.10–16 It has been suggested that growing tumors induce thrombocytosis by secretion of different cytokines and growth factors.17–20 But platelets might also influence tumor growth. Increased activation, adhesion, and aggregation of circulating platelets within pathologically altered and prothrombotic tumor vessels, with subsequent secretion of different growth factors from activated platelets, have been suggested to result in increased angiogenesis and tumor growth rates.21,22 It remains questionable whether thrombocytosis is simply an end result of growth factors secreted by tumor cells or whether thrombocytosis is an event that directly increases the risk of disease spread and worsened prognosis.
For glioblastoma, the impact of various histopathological,23–25 genetic,26,27 and clinical28–30 prognostic factors has been documented in several studies. To our knowledge, there are no studies investigating the prognostic significance of thrombocytosis as a survival factor in patients with glioblastoma. This retrospective study is the first to assess the influence of pretreatment thrombocytosis on survival in these patients.
Materials and Methods
Patients and Parameters
For this retrospective study, we tried to identify as many patients as possible diagnosed with glioblastoma multiforme (GBM) between October 1999 and July 2004 by searching medical records in the archives of the Mannheim University Hospital and the Schleswig-Holstein University Hospital (Campus Luebeck). A total of 187 patients treated for GBM could be identified. Of these 187 patients, 26 could not be included in the study because they were lost to follow-up and/or no definite information regarding survival time could be obtained. In three cases, relevant preoperative laboratory investigations were missing, and in five cases, the patient chart was missing in the archive. Finally, the medical records of the remaining 153 patients with histologically confirmed GBM (WHO glioma grade IV), who underwent treatment at either participating center, were included for retrospective analysis.
The data assessed from the patient charts included gender, patient age, preoperative platelet count (platelets/nl), fibrinogen (g/l), prothrombin time (%), activated partial thromboplastin time (aPTT; seconds), KPS, tumor volume (ml), and other details of the patient history such as smoking habits, treatment modalities (complete/incomplete tumor resection based on the surgeon’s report, biopsy only, postoperative radiation therapy [RT] with or without additional chemotherapy [CT]), and postoperative survival time (in months, with 95% confidence interval [95% CI]). If patients did not undergo complete or incomplete tumor resection but only biopsy, the survival time after histopathological diagnosis of GBM was used for statistical analyses.
All preoperative hematological analyses were taken within 14 days prior to tumor resection or biopsy. Thrombocytosis was considered as a platelet count greater than 400/nl, in accord with the literature.4,6,31 Patients with a history of myeloproliferative disorders, acute inflammatory disease, or splenectomy were not included in this study.
Analysis of Preoperative Tumor Volume
If available at the time of data analysis, digitally stored preoperative computed tomography or MR images were used for volumetric analyses of tumors using Argus software (Argus VA 60C; Siemens, Erlangen, Germany) as supplied with one of the hospital’s MRI scanners. Only preoperative images acquired and digitally stored in one of the study centers were considered for volumetric analyses. Preoperative images acquired off-site were not available for analysis.
Statistical Analysis
Gross total resection has been found to be associated with significantly improved survival compared with biopsy only.32–34 In addition to the group containing all patients, we decided to analyze a subpopulation of patients with complete or incomplete tumor resection and RT with or without additional CT (n = 98) in order to evaluate the specific characteristics of this group.
Differences in clinical factors and hematological parameters between patients with and without thrombocytosis were examined with the chi-square, Mann-Whitney, Fisher’s exact, and two-sample t-test according to the type of underlying parameter. The survival time was estimated by the Kaplan-Meier method, in which the response variable (survival time) was related to one explanatory variable (e.g., platelet count). Kaplan-Meier curves were compared with the log-rank test. The Cox proportional hazards model was used to assess the significance of potential prognostic factors as predictive parameters for patient survival. After univariate and multivariate analysis of all potential confounding factors, we tried to identify the best prognostic model with a relatively small number of variables. This procedure was chosen to make sure that none of the important factors was omitted. Effects with a p value equal to or less than 0.05 were considered to be statistically significant.
Results
All Patients
The records of 153 patients (63 female, 90 male) with histopathologically confirmed glioblastoma were analyzed. Of these 153 patients, 89 were treated at the Mannheim University Hospital and 64 at the Schleswig-Holstein University Hospital (Campus Luebeck). Clinical parameters and associations between presence and absence of thrombocytosis of all patients are presented in Table 1.
Table 1.
Clinical parameters and associations between presence and absence of thrombocytosis in all patients with glioblastoma (n = 153)
| Parameter | < 400 Platelets/nl (n = 124) | > 400 Platelets/nl (n = 29) | Test | PValue |
|---|---|---|---|---|
| Age (years) | 64.9 ± 11.1 (n =124) | 64.8 ± 10.4 (n =29) | t-test | 0.9498 |
| KPS | 80 (40–100; n =124) | 80 (20–90; n =26) | Mann-Whitney test | 0.7636 |
| Tumor volume (ml) | 24.8 ± 21.8 (n =55) | 38.9 ± 22.5 (n =12) | t-test | 0.0481 |
| Platelet count (per nl) | 276 ± 62 (n = 124) | 451 ± 54 (n = 29) | t-test | < 0.0001 |
| Hemoglobin (g/100 ml) | 14.5 ± 1.4 (n =124) | 14.3 ± 1.6 (n =29) | t-test | 0.3978 |
| aPTT (s) | 23.6 ± 3.2 (n =117) | 24.4 ± 3.4 (n =28) | t-test | 0.2471 |
| Prothrombin time (%) | 98.8 ± 13.8 (n =122) | 99.0 ± 10.3 (n =29) | t-test | 0.9377 |
| Fibrinogen (g/l) | 2.9 ± 1.0 (n = 79) | 3.7 ± 1.7 (n = 23) | t-test | 0.0323 |
| Smokers | 16 (14.3%) | 5 (18.5%) | Fisher’s exact test | 0.5588 |
| Nonsmokers | 96 (85.7%) | 22 (81.5%) | ||
| Female | 49 (39.5%) | 14 (48.3%) | Chi-square test | 0.3882 |
| Male | 75 (60.5%) | 15 (51.7%) | ||
| Biopsy only | 29 (23.4%) | 11 (38.0%) | Chi-square test | 0.0392 |
| Incomplete resection | 24 (19.4%) | 9 (31.0%) | ||
| Complete resection | 71 (57.3%) | 9 (31.0%) | ||
| No RT or CT | 17 (13.7%) | 9 (31.0%) | Chi-square test | 0.079 |
| RT only | 55 (44.4%) | 11 (38.0%) | ||
| RT and CT | 52 (41.9%) | 9 (31.0%) |
Abbreviations: aPTT, activated partial thromboplastin time; RT, radiation therapy, CT, chemotherapy. Note that for these analyses, all patients with glioblastoma, regardless of their further therapy or other parameters, were included. All quantitative parameters are given as mean ± 1 SD; KPS is given as median and range; the qualitative parameters are expressed in frequencies or number of patients (%). The degree of tumor resection was based on the surgeon’s report.
Macroscopically complete resection (based on the surgeons report) was achieved in 80 patients (52.3%), while a partial resection was performed in 33 patients (21.6%). Forty patients (26.1%) were considered inoperable due to tumor location in inaccessible areas or because of the patient’s poor condition.
Of all 153 patients, 127 patients (83.0%) were treated by postoperative RT, performed as three-dimensional irradiation with a median dose of 60 Gy (mean ± 1 SD, 56.0 ± 6.4 Gy). Of these 127 patients, 61 received additional CT (temozolomide = 28, temozolomide + rofecoxib = 12; imatinib = 11; nimustine hydrochloride = 7; other chemotherapies = 3). No patient was treated by CT alone. In 26 of all 153 patients (17.0%), no radiation or CT was performed. Of these 26 patients, 11 underwent biopsy only, 10 underwent incomplete tumor resection, and only 5 underwent macroscopically complete tumor resection. Reasons to refrain from RT and/or CT were refusal of patients to undergo RT and/or CT, a poor postoperative performance status, or the fact that patients were considered primarily inoperable and presented with rapidly deteriorating performance. Analysis of the medical records revealed 21 of the patients to be chronic smokers (15.1%), while 118 patients (84.9%) identified themselves as nonsmokers. For the remaining 14 patients, no information on smoking behavior was available. Twenty-nine patients (19.0%) presented with preoperative thrombocytosis. The mean (± 1 SD) preoperative tumor volume was acquired in 67 patients (27.3 ± 22.4 ml). Platelet counts were higher in female (n = 63) than in male (n = 90) patients (326 ± 97 vs. 297 ± 86; p = 0.0555, t-test). Nevertheless, platelet count retained significance in multivariate analysis as a factor influencing survival, whereas gender did not affect survival time in univariate or multivariate analyses (p = 0.7081, log-rank test).
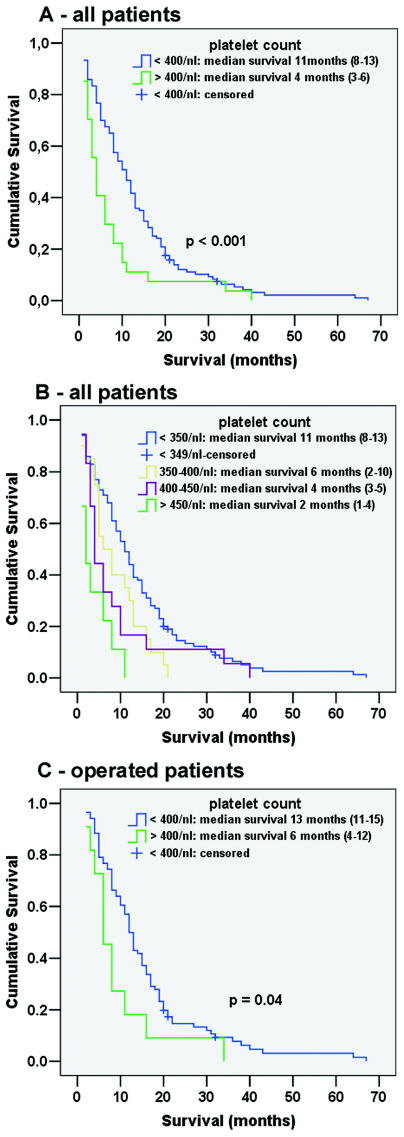
The median survival time for all 153 patients was nine months (95% CI, 7–11). Univariate analysis (log-rank test) revealed preoperative thrombocytosis to be significantly associated with reduced survival times (4 months; 95% CI, 3–6 months) compared to patients with normal platelet counts (11 months; 95% CI, 8–13 months; p = 0.0006; Fig. 1A). Fig. 1B indicates how platelet levels affect survival time and shows that even elevated platelet levels still within the normal range (350–400 platelets/nl) are associated with shorter survival. Continuous variables significantly associated with shorter survival time were elevated preoperative platelet count (p = 0.0002), elevated fibrinogen levels (p = 0.0178), higher age (p = 0.0001), decreased prothrombin time (p = 0.0535), and prolonged aPTT (p = 0.0340). There was a trend that larger preoperative tumor volume likewise was associated with shorter survival (p = 0.0545). In our population, the KPS showed no significant association with survival time (p = 0.1356) when applied as a quantitative variable. A highly significant difference in median survival time was detected when comparing a group of patients with a KPS of 60 or less (5.5 months; 95% CI, 5–9 months) with patients with a KPS of 70 or higher (11 months; 95% CI, 8–13 months; p = 0.0047).
Fig 1.
Effect of elevated platelet counts on survival duration in patients with glioblastoma: survival in months (95% confidence interval [95% CI]). (A) Patients with glioblastoma with preoperative thrombocytosis had a statistically significantly shorter overall survival (log-rank test). The composition of the patient groups is described in Table 1. (B) Overall survival of all patients with glioblastoma after creating four strata of platelet counts. Note that patients with platelet counts elevated within the normal range (350–400 platelets/nl) had a significantly reduced survival time compared to patients with a platelet count below 350/nl (p = 0.022). Patients with thrombocytosis below 450 platelets/nl can be expected to have a median survival time of four months (95% CI, 3–5 months) compared to two months (95% CI, 1–4 months) in patients with thrombocytosis greater than 450 platelets/nl (p = 0.057). (C) Overall survival of only patients with complete or incomplete tumor resection and subsequent radiation therapy, with or without additional chemotherapy. The composition of this patient group is described in Table 3.
After univariate and multivariate analyses of all potential confounding factors, we identified the best prognostic model with a relatively small number of variables, to make sure that none of the important factors was omitted in the model. After multivariate analysis, the following variables retained significance as prognostic factors (Table 2): preoperative platelet count (p < 0.0001), age (p = 0.0002), preoperative prothrombin time (p = 0.0163), and aPTT (p = 0.0263).
Table 2.
Multivariate Cox proportional hazards analysis of potential prognostic factors on overall survival in patients with glioblastoma
| Variable | Unit | Hazard Ratio | 95% Confidence Interval | PValue |
|---|---|---|---|---|
| Preoperative platelet count | 100 counts | 1.597 | 1.427–1.747 | <0.0001 |
| Age | 10 years | 1.406 | 1.283–1.541 | 0.0002 |
| Preoperative prothrombin time | 10% | 0.868 | 0.818–0.921 | 0.0163 |
| aPTT | 1 s | 1.069 | 1.037–1.102 | 0.0263 |
Abbreviation: aPTT, activated partial thromboplastin time. For this analysis, all patients with glioblastoma, regardless of their further therapy or other parameters, were included. Age, preoperative platelet count, and preoperative prothrombin time were analyzed as continuous variables.
We additionally analyzed the correlation of preoperative platelet count and other preoperative clinical parameters using Spearman’s rho (correlation coefficient, p value). Preoperative platelet count correlated positively with tumor volume (rs = 0.305, p = 0.0122, n = 67) and negatively with degree of tumor resection (rs = −0.233, p = 0.0037, n = 153). Other significant correlations found were tumor volume (ml) and survival (rs = −0.271, p = 0.028, n = 66), degree of tumor resection and preoperative fibrinogen levels (rs = −0.342, p = 0.0004, n = 102), age and degree of tumor resection (rs = −0.219, p = 0.0067, n = 153), and age and KPS (rs = −0.179, p = 0.0304, n = 146).
Definite information on cause of death (COD) was available in eight patients: venous thrombosis (n = 1), pneumonia and sepsis (n = 3), intracerebral hemorrhage (n = 2), and basilar and median cerebral artery thrombosis (each n = 1). Both patients with intracerebral artery thrombosis belonged to the group of patients with preoperative thrombosis and died within the first month after the operation. The patient with venous thrombosis died five months after diagnosis of GBM and initially presented with a platelet count of 388/nl.
Factors Influencing Survival in Operated Patients with Subsequent RT with or without CT
This subpopulation consisted of 98 patients who underwent complete or incomplete tumor resection with subsequent RT with or without additional CT. Non-operated patients and patients with complete or incomplete tumor resection but without subsequent RT were excluded from this subanalysis to better control the factor of postoperative RT. Table 3 shows the associations between the presence or absence of thrombocytosis and clinical parameters in these patients and demonstrates that both groups are approximately balanced for all factors, including degree of tumor resection. Platelet count and tumor volume were the only unbalanced factors and again correlated positively with each other (rs = 0.370, p = 0.0173, n = 41). Median survival time in this group was 12 months (95% CI, 10–14 months).
Table 3.
Clinical parameters and associations between presence and absence of thrombocytosis in all operated patients with subsequent radiation therapy with or without additional chemotherapy
| Parameter | < 400 Platelets/nl (n = 87) | > 400 Platelets/nl (n = 11) | Test | PValue |
|---|---|---|---|---|
| Age (years) | 63.3 ± 11.1 (n =87) | 65.8 ± 6.4 (n = 11) | t-test | 0.4620 |
| KPSa | 80 (50–100; n =86) | 80 (50–90; n =11) | Mann-Whitney test | 0.6618 |
| Tumor volume (ml) | 23.5 ± 20.3 (n =37) | 48.7 ± 23.1 (n =4) | t-test | 0.0248 |
| Platelet count (per nl) | 275 ± 65 (n =87) | 440 ± 42 (n =11) | t-test | < 0.0001 |
| Hemoglobin (g/100 ml) | 14.5 ± 1.4 (n = 87) | 14.2 ± 1.7 (n = 11) | t-test | 0.6376 |
| aPTT (s) | 23.2 ± 2.9 (n = 81) | 24.4 ± 2.7 (n = 10) | t-test | 0.2069 |
| Prothrombin time (%) | 99.8 ± 11.0 (n =86) | 97.5 ± 13.4 (n =11) | t-test | 0.5382 |
| Fibrinogen (g/l) | 2.7 ± 1.0 (n = 51) | 3.2 ± 1.5 (n = 7) | t-test | 0.4248 |
| Smokers | 12 (15%) | 3 (30%) | Fisher’s exact test | 0.3610 |
| Nonsmokers | 68 (85%) | 7 (70%) | ||
| Female | 36 (41.4%) | 5 (45.5%) | Fisher’s exact test | 1.0000 |
| Male | 51 (58.6%) | 6 (54.5%) | ||
| Incomplete resection | 20 (23.0%) | 3 (27.3%) | Fisher’s exact test | 0.7164 |
| Complete resection | 67 (77.0%) | 8 (72.7%) | ||
| RT only | 43 (49.4%) | 4 (36.4%) | Chi-square test | 0.4139 |
| RT and CT | 44 (50.6%) | 7 (63.6%) |
Abbreviations: aPTT, activated partial thromboplastin time; RT, radiation therapy, CT, chemotherapy. Nonoperated patients or operated patients without subsequent RT were excluded from this analysis. All quantitative parameters are given as mean ± 1 SD or number of patients (%); KPS is given as median and range; the qualitative parameters are expressed in frequencies.
Univariate analysis (log-rank test) revealed the following factors to be significantly associated with survival time: incomplete tumor resection (8 months; 95% CI, 6–10 months) versus complete tumor resection (13 months; 95% CI, 12–16 months; p = 0.0077), and pre-operative thrombocytosis (6 months; 95% CI, 4–12 months) versus normal platelet count (13 months; 95% CI, 11–15 months; p = 0.0359; Fig. 1C). A KPS of 60 or less was clearly, although not significantly, associated with shorter survival (9 months; 95% CI, 5–14 months) compared to a score of 70 or higher (13 months; 95% CI, 11–16 months; p = 0.0871). There was a tendency for RT with additional CT to prolong survival times (14 months; 95% CI, 12–17 months) compared to RT alone (8.5 months; 95% CI, 6–12 months), and for survival time to be prolonged in patients presenting with a preoperative tumor volume of less than 20 ml (14 months; 95% CI, 11–17 months) compared to patients with tumor volumes exceeding 20 ml (7 months; 95% CI, 4–10 months). These differences, however, were not statistically significant (p = 0.1173 and p = 0.16, respectively).
Continuous variables associated with shorter survival time were higher age (p = 0.0083), elevated preoperative platelet counts (p = 0.018), and elevated fibrinogen levels (p = 0.087). In this patient population, KPS and aPTT failed to retain significant influence on patient survival time.
Multivariate analysis (Cox proportional hazards model) rendered age (p = 0.0115), prothrombin time (p = 0.0228), degree of tumor resection (p = 0.0349), and elevated preoperative platelet counts (p = 0.0384) to be valuable predictors of survival time in this group of operated patients with subsequent RT with or without additional CT (Table 4). Because the degree of tumor resection is based on the surgeon’s report, an inadequate estimation of the degree of tumor resection in this group cannot be completely ruled out. Furthermore, few studies have convincingly shown an advantage to gross total versus partial resection. Lacroix et al.35 is a good example of the latter, but even its authors concede that some degree of selection bias is likely present. The results describing the effects of the degree of tumor resection thus require critical interpretation by the reader.
Table 4.
Multivariate Cox proportional hazards analysis of potential prognostic factors on survival in operated patients (complete or incomplete tumor resection) with subsequent radiation therapy (with or without additional chemotherapy)
| Variable | Unit | Hazard Ratio | 95% Confidence Interval | PValue |
|---|---|---|---|---|
| Age | 10 years | 1.316 | 1.180–1.438 | 0.0115 |
| Preoperative prothrombin time | 10% | 0.785 | 0.706–0.873 | 0.0228 |
| Preoperative platelet count | 100 platelets | 1.327 | 1.157–1.521 | 0.0384 |
| Degree of tumor resection | — | 0.562 | 0.428–0.738 | 0.0349 |
Age and preoperative prothrombin time were analyzed as continuous variables; degree of tumor resection was analyzed as nominal variable. The degree of tumor resection was based on the surgeon’s report and defined as complete or incomplete tumor resection.
We additionally analyzed the influence of thrombocytosis on survival time in a subpopulation of all patients with complete tumor resection (n = 75). We found a striking disadvantage regarding median survival time in patients with preoperative thrombocytosis (n = 8) compared to patients without thrombocytosis (n = 67; 13 months vs. 18 months, respectively; log-rank test: p = 0.235). This finding, however, was not significant in this subpopulation, which is most likely due to the smaller number of patients.
Discussion
The relation of thrombocytosis with malignant disorders has been known for more than a century.36 Higher platelet counts are known to be an independent poor prognostic marker associated with advanced tumor stage and decreased survival rates in solid tumors.3,6,9,14,31,37 The frequency of thrombocytosis and thrombocyte counts vary in malignant diseases. The ranges of frequencies for thrombocytosis as previously reported are 9.5%–38% in gynecological cancer,11,16,37 13%–60% in bronchial cancer,5,38,39 and 56.8% in renal cancer.6 In our study, 19% of all patients with GBM presented with thrombocytosis. Likewise, our results demonstrate pretreatment thrombocytosis to be associated not only with reduced survival time but also with larger preoperative tumor volumes, which similarly has been reported for hepatocellular carcinoma.40
Thrombocytosis is most likely a result of tumor burden. Excessive amounts of cytokines were found to be associated with thrombocytosis in solid cancers. Interleukin-6 (IL-6), IL-1, vascular endothelial growth factor (VEGF), macrophage colony-stimulating factor, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and tumor necrosis factor-α are some of the cytokines that have been studied in malignancy-associated thrombocytosis.41–45 Furthermore, platelet counts have been found to correlate well with blood VEGF levels in cancer.44 Some of the cytokines associated with increased platelet levels have also been found to be secreted by GBM or to be increased in the blood of patients with GBM. Choi et al.46 and van Meir et al.47 reported increased IL-6 levels in GBM, while Valter et al.48 and Ross et al.49 reported increased levels of IL-1 in gliomas. Another study found IL-1α and IL-1β to induce the production of GM-CSF and G-CSF in human astroglial cell lines.50 These findings, and the fact that, compared to a normal population with a mean platelet count of 250/nl,51,52 our patients present with an obviously increased mean platelet count of 309 platelets/nl, which positively correlates with tumor volume, suggest thrombocytosis results from tumor growth.
Although an influence of tumor growth on thrombocytosis is generally accepted, the reverse remains unclear: does thrombocytosis influence tumor growth and angiogenesis? It has been proposed that the release of growth factors from platelets activated within prothrombotic tumor microcirculation increases angiogenesis and tumor growth. If so, this could result in a vicious circle, whereby tumor growth induces thrombocytosis, and elevated platelet counts lead to increased tumor growth and angiogenesis. Our recent results have demonstrated that platelet-derived growth factors as well as VEGF, which is also stored in platelets, exert strong chemotactic effects on the migration of human cerebral micro-vascular endothelial cells, which is an essential step in angiogenesis.53 Our ongoing unpublished investigations indicate a strong influence of platelets on glioma and endothelial cell growth and migration in vitro, but there also have been contradicting results from in vivo studies of intratumoral microthrombosis and platelet adhesion. Manegold et al.54 assessed platelet adherence by intravital fluorescence video microscopy of Lewis lung carcinoma (LLC 1) and fibrosarcoma (BFS 1) using a dorsal skinfold chamber model and found only slightly increased rolling of fluorescence-labeled platelets and no obvious platelet adhesion. These results, however, might not represent the physiological situation, since they were observed in a highly artificial setting with fluorescence-labeled platelets and short observation periods.
If thrombocytosis is associated with platelet-promoted tumor growth, then selective and controlled reduction of platelet counts (e.g., using cytapheresis) to normal levels, without increasing the risk of intratumoral hemorrhage, might provide a treatment option in patients with significantly elevated platelet counts. Because of the increased risk for intratumoral hemorrhage, antiplatelet drugs may not be a good solution. Nevertheless, Arrieta et al.55 treated C6 glioma-bearing rats with acetylsalicylic acid (ASA) and found a reduction in tumor size after administration of low-dose ASA, although higher doses seemed paradoxically to promote tumor growth. Other groups have also investigated the influence of inhibitors of the arachidonic acid metabolism on tumor cell growth and migration.56,57 These studies demonstrated that inhibitors of cyclooxygenases and thromboxane synthase block the migration of endothelial cells58 and malignant glioma cells, induce apoptosis, and sensitize migration-arrested cells to drug-induced apoptosis.56,59,60
The significant correlation between increased prothrombin time and shortened survival observed in this study cannot be easily explained, especially because mean prothrombin time in both patient groups (with and without thrombocytosis) was within the normal range and no significant differences could be observed. We also investigated the effect of smoking habits on survival in our patient population, because smoking has been controversially discussed to have prothrombotic and platelet-activating effects.61–63 However, no significant correlations with platelet count or patient survival were found in our study.
Regarding COD, we have definite information only for eight of our patients. In the remaining patients, no autopsy was performed and no definite COD could be extracted from patients’ charts. How many of our patients definitely died as a direct result of tumor growth is unknown. Because COD in patients with supratentorial glioblastoma has been found to be multifactorial,64 the actual COD is often difficult to determine. In three of the patients with known COD, however, possible thrombosis-related mechanisms (median cerebral and basilar artery thrombosis and venous thrombosis) were identified as COD.
In conclusion, our retrospective study identified pre-treatment thrombocytosis as an independent risk factor for survival time in patients with glioblastoma. Because patients with GBM present with increased platelet counts compared to a normal population, increased platelet levels are likely to be the result of tumor growth. Whether increased platelet counts increase tumor growth and angiogenesis remains open for discussion.
Acknowledgments
We thank Thomas Nebe, M.D. (Institute of Clinical Chemistry, Mannheim University Hospital), for his assistance in acquisition of patient data.
References
- 1.Herndon JE, Green MR, Chahinian AP, Corson JM, Suzuki Y, Vogelzang NJ. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest. 1998;113:723–731. doi: 10.1378/chest.113.3.723. [DOI] [PubMed] [Google Scholar]
- 2.Engan T, Hannisdal E. Blood analyses as prognostic factors in primary lung cancer. Acta Oncol. 1990;29:151–154. doi: 10.3109/02841869009126536. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9:1826–1830. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- 4.Costantini V, Zacharski LR, Moritz TE, Edwards RL. The platelet count in carcinoma of the lung and colon. Thromb Haemost. 1990;64:501–505. [PubMed] [Google Scholar]
- 5.Silvis SE, Turkbas N, Doscherholmen A. Thrombocytosis in patients with lung cancer. JAMA. 1970;211:1852–1853. [PubMed] [Google Scholar]
- 6.Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA. Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma. BJU Int. 2000;86:203–207. doi: 10.1046/j.1464-410x.2000.00792.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Keefe SC, Marshall FF, Issa MM, Harmon MP, Petros JA. Thrombocytosis is associated with a significant increase in the cancer specific death rate after radical nephrectomy. J Urol. 2002;168:1378–1380. doi: 10.1016/S0022-5347(05)64453-9. [DOI] [PubMed] [Google Scholar]
- 8.Monreal M, Fernandez-Llamazares J, Pinol M, et al. Platelet count and survival in patients with colorectal cancer—a preliminary study. Thromb Haemost. 1998;79:916–918. [PubMed] [Google Scholar]
- 9.Shimada H, Oohira G, Okazumi S, et al. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surg. 2004;198:737–741. doi: 10.1016/j.jamcollsurg.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Li AJ, Madden AC, Cass I, Leuchter RS, Lagasse LD, Karlan BY. The prognostic significance of thrombocytosis in epithelial ovarian carcinoma. Gynecol Oncol. 2004;92:211–214. doi: 10.1016/j.ygyno.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez E, Lavine M, Dunton CJ, Gracely E, Parker J. Poor prognosis associated with thrombocytosis in patients with cervical cancer. Cancer. 1992;69:2975–2977. doi: 10.1002/1097-0142(19920615)69:12<2975::aid-cncr2820691218>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez E, Donohue KA, Anderson LL, Heller PB, Stehman FB. The significance of thrombocytosis in patients with locally advanced cervical carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78:137–142. doi: 10.1006/gyno.2000.5838. [DOI] [PubMed] [Google Scholar]
- 13.Chalas E, Welshinger M, Engellener W, Chumas J, Barbieri R, Mann WJ. The clinical significance of thrombocytosis in women presenting with a pelvic mass. Am J Obstet Gynecol. 1992;166:974–977. doi: 10.1016/0002-9378(92)91375-k. [DOI] [PubMed] [Google Scholar]
- 14.Zeimet AG, Marth C, Muller-Holzner E, Daxenbichler G, Dapunt O. Significance of thrombocytosis in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1994;170:549–554. doi: 10.1016/s0002-9378(94)70225-x. [DOI] [PubMed] [Google Scholar]
- 15.Taucher S, Salat A, Gnant M, et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb Haemost. 2003;89:1098–1106. [PubMed] [Google Scholar]
- 16.Hefler L, Mayerhofer K, Leibman B, et al. Tumor anemia and thrombocytosis in patients with vulvar cancer. Tumour Biol. 2000;21:309–314. doi: 10.1159/000030136. [DOI] [PubMed] [Google Scholar]
- 17.Nakazaki H. Preoperative and postoperative cytokines in patients with cancer. Cancer. 1992;70:709–713. doi: 10.1002/1097-0142(19920801)70:3<709::aid-cncr2820700328>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Wu CW, Wang SR, Chao MF, et al. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol. 1996;91:1417–1422. [PubMed] [Google Scholar]
- 19.Lidor YJ, Xu FJ, Martinez-Maza O, et al. Constitutive production of macrophage colony-stimulating factor and interleukin-6 by human ovarian surface epithelial cells. Exp Cell Res. 1993;207:332–329. doi: 10.1006/excr.1993.1200. [DOI] [PubMed] [Google Scholar]
- 20.Kabir S, Daar GA. Serum levels of interleukin-1, interleukin-6 and tumour necrosis factor-alpha in patients with gastric carcinoma. Cancer Lett. 1995;95:207–212. doi: 10.1016/0304-3835(95)03895-4. [DOI] [PubMed] [Google Scholar]
- 21.Pinedo HM, Verheul HM, D’Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352:1775–1777. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- 22.Kisucka J, Butterfield CE, Duda DG, et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci U S A. 2006;103:855–860. doi: 10.1073/pnas.0510412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher BJ, Naumova E, Leighton CC, et al. Ki-67: a prognostic factor for low-grade glioma? Int J Radiat Oncol Biol Phys. 2002;52:996–1001. doi: 10.1016/s0360-3016(01)02720-1. [DOI] [PubMed] [Google Scholar]
- 24.Fuse T, Tanikawa M, Nakanishi M, et al. p27Kip1 expression by contact inhibition as a prognostic index of human glioma. J Neurochem. 2000;74:1393–1399. doi: 10.1046/j.1471-4159.2000.0741393.x. [DOI] [PubMed] [Google Scholar]
- 25.Muracciole X, Romain S, Dufour H, et al. PAI-1 and EGFR expression in adult glioma tumors: toward a molecular prognostic classification. Int J Radiat Oncol Biol Phys. 2002;52:592–598. doi: 10.1016/s0360-3016(01)02699-2. [DOI] [PubMed] [Google Scholar]
- 26.McDonald FE, Ironside JW, Gregor A, et al. The prognostic influence of bcl-2 in malignant glioma. Br J Cancer. 2002;86:1899–904. doi: 10.1038/sj.bjc.6600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rustia A, Wierzbicki V, Marrocco L, Tossini A, Zamponi C, Lista F. Is exon 5 of the PTEN/MMAC1 gene a prognostic marker in anaplastic glioma? Neurosurg Rev. 2001;24:97–102. doi: 10.1007/pl00014589. [DOI] [PubMed] [Google Scholar]
- 28.Winger MJ, Macdonald DR, Cairncross JG. Supratentorial anaplastic gliomas in adults: the prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989;71:487–493. doi: 10.3171/jns.1989.71.4.0487. [DOI] [PubMed] [Google Scholar]
- 29.Lote K, Egeland T, Hager B, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15:3129–3140. doi: 10.1200/JCO.1997.15.9.3129. [DOI] [PubMed] [Google Scholar]
- 30.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30(6 suppl 19):10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda M, Furukawa H, Imamura H, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9:287–291. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- 32.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 33.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. J Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 34.Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67:121–132. [PubMed] [Google Scholar]
- 35.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 36.Riess L. Zur pathologischen anatomie des blutes. Arch Anat Physiol Wissensch Med. 1872;39:237–249. [Google Scholar]
- 37.Lavie O, Comerci G, Daras V, Bolger BS, Lopes A, Monaghan JM. Thrombocytosis in women with vulvar carcinoma. Gynecol Oncol. 1999;72:82–86. doi: 10.1006/gyno.1998.5225. [DOI] [PubMed] [Google Scholar]
- 38.Gislason T, Nou E. Sedimentation rate, leucocytes, platelet count and haemoglobin in bronchial carcinoma: an epidemiological study. Eur J Respir Dis. 1985;66:141–146. [PubMed] [Google Scholar]
- 39.Pedersen LM, Milman N. Diagnostic significance of platelet count and other blood analyses in patients with lung cancer. Oncol Rep. 2003;10:213–216. [PubMed] [Google Scholar]
- 40.Hwang SJ, Luo JC, Li CP, et al. Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:2472–2477. doi: 10.3748/wjg.v10.i17.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 42.Hollen CW, Henthorn J, Koziol JA. Serum interleukin-6 levels in patients with thrombocytosis. Leuk Lymphoma. 1992;8:235–241. doi: 10.3109/10428199209054910. [DOI] [PubMed] [Google Scholar]
- 43.Tefferi A, Ho TC, Ahmann GJ, Katzmann JA, Greipp PR. Plasma inter-leukin-6 and C-reactive protein levels in reactive versus clonal thrombocytosis. Am J Med. 1994;97:374–378. doi: 10.1016/0002-9343(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 44.Salgado R, Vermeulen PB, Benoy I, et al. Platelet number and interleukin-6 correlate with VEGF but not with bFGF serum levels of advanced cancer patients. Br J Cancer. 1999;80:892–897. doi: 10.1038/sj.bjc.6690437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeuchi E, Ito M, Mori M, et al. Lung cancer producing interleukin-6. Intern Med. 1996;35:212–214. doi: 10.2169/internalmedicine.35.212. [DOI] [PubMed] [Google Scholar]
- 46.Choi C, Gillespie GY, Van Wagoner NJ, Benveniste EN. Fas engagement increases expression of interleukin-6 in human glioma cells. J Neurooncol. 2002;56:13–19. doi: 10.1023/a:1014467626314. [DOI] [PubMed] [Google Scholar]
- 47.Van Meir E, Sawamura Y, Diserens AC, Hamou MF, de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990;50:6683–6688. [PubMed] [Google Scholar]
- 48.Valter MM, Wiestler OD, Pietsche T. Differential control of VEGF synthesis and secretion in human glioma cells by IL-1 and EGF. Int J Dev Neurosci. 1999;17:565–577. doi: 10.1016/s0736-5748(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 49.Ross HJ, Antoniono RJ, Buckmeier JA, Redpath JL. Variable expression of IL-1 beta has minimal effect on the radiation sensitivity of three human glioma cell lines. Int J Radiat Biol. 1994;66:785–791. [PubMed] [Google Scholar]
- 50.Tweardy DJ, Glazer EW, Mott PL, Anderson K. Modulation by tumor necrosis factor-alpha of human astroglial cell production of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) J Neuroimmunol. 1991;32:269–278. doi: 10.1016/0165-5728(91)90197-f. [DOI] [PubMed] [Google Scholar]
- 51.Brecher G, Schneiderman M, Cronkite EP. The reproducibility and constancy of the platelet count. Am J Clin Pathol. 1953;23:15–26. doi: 10.1093/ajcp/23.1.15. [DOI] [PubMed] [Google Scholar]
- 52.Karpatkin S, Garg SK, Siskind GW. Autoimmune thrombocytopenic purpura and the compensated thrombocytolytic state. Am J Med. 1971;51:1–4. doi: 10.1016/0002-9343(71)90317-2. [DOI] [PubMed] [Google Scholar]
- 53.Brockmann MA, Ulbricht U, Gruner K, Fillbrandt R, Westphal M, Lamszus K. Glioblastoma and cerebral microvascular endothelial cell migration in response to tumor-associated growth factors. Neurosurgery. 2003;52:1391–1399. doi: 10.1227/01.neu.0000064806.87785.ab. [DOI] [PubMed] [Google Scholar]
- 54.Manegold PC, Hutter J, Pahernik SA, Messmer K, Dellian M. Platelet-endothelial interaction in tumor angiogenesis and microcirculation. Blood. 2003;101:1970–1976. doi: 10.1182/blood.V101.5.1970. [DOI] [PubMed] [Google Scholar]
- 55.Arrieta O, Guevara P, Reyes S, Palencia G, Rivera E, Sotelo J. Paradoxical effect of aspirin on the growth of C6 rat glioma and on time of development of ENU-induced tumors of the nervous system. J Cancer Res Clin Oncol. 2001;127:681–686. doi: 10.1007/s004320100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giese A, Hagel C, Kim EL, et al. Thromboxane synthase regulates the migratory phenotype of human glioma cells. Neuro-Oncology. 1999;1:3–13. doi: 10.1093/neuonc/1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aas AT, Tonnessen TI, Brun A, Salford LG. Growth inhibition of rat glioma cells in vitro and in vivo by aspirin. J Neurooncol. 1995;24:171–180. doi: 10.1007/BF01078487. [DOI] [PubMed] [Google Scholar]
- 58.Jantke J, Ladehoff M, Kurzel F, Zapf S, Kim E, Giese A. Inhibition of thearachidonic acid metabolism blocks endothelial cell migrationand induces apoptosis. Acta Neurochir (Wien) 2004;146:483–494. doi: 10.1007/s00701-004-0238-z. [DOI] [PubMed] [Google Scholar]
- 59.Yoshizato K, Zapf S, Westphal M, Berens ME, Giese A. Thromboxane synthase inhibitors induce apoptosis in migration-arrested glioma cells. Neurosurgery. 2002;50:343–354. doi: 10.1097/00006123-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 60.Kurzel F, Hagel C, Zapf S, Meissner H, Westphal M, Giese A. Cyclooxygenase inhibitors and thromboxane synthase inhibitors differentially regulate migration arrest, growth inhibition and apoptosis in human glioma cells. Acta Neurochir (Wien) 2002;144:71–87. doi: 10.1007/s701-002-8276-9. [DOI] [PubMed] [Google Scholar]
- 61.Brockmann MA, Beythien C, Magens MM, Wilckens V, Kuehnl P, Gutensohn K. Platelet hemostasis capacity in smokers: in vitro function analyses with 3.2% citrated whole blood. Thromb Res. 2001;104:333–342. doi: 10.1016/s0049-3848(01)00382-6. [DOI] [PubMed] [Google Scholar]
- 62.Morita H, Ikeda H, Haramaki N, Eguchi H, Imaizumi T. Only two-week smoking cessation improves platelet aggregability and intra-platelet redox imbalance of long-term smokers. J Am Coll Cardiol. 2005;45:589–594. doi: 10.1016/j.jacc.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 63.Nair S, Kulkarni S, Camoens HM, Ghosh K, Mohanty D. Changes in platelet glycoprotein receptors after smoking—a flow cytometric study. Platelets. 2001;12:20–26. doi: 10.1080/09537100120046020. [DOI] [PubMed] [Google Scholar]
- 64.Silbergeld DL, Rostomily RC, Alvord EC., Jr The cause of death in patients with glioblastoma is multifactorial: clinical factors and autopsy findings in117 cases of supratentorial glioblastoma inadults. J Neurooncol. 1991;10:179–185. doi: 10.1007/BF00146880. [DOI] [PubMed] [Google Scholar]