Abstract
The anatomic location of a glioma influences prognosis and treatment options. The aim of our study was to describe the distribution of gliomas in different anatomic areas of the brain. A representative population-based sample of 331 adults with glioma was used for preliminary analyses. The anatomic locations for 89 patients from a single center were analyzed in more detail from radiologic imaging and recorded on a three-dimensional 1 × 1 × 1– cm grid. The age-standardized incidence rate of gliomas was 4.7 per 100,000 person-years. The most frequent subtypes were glioblastoma (47%) and grade II–III astrocytoma (23%), followed by oligodendroglioma and mixed glioma. The gliomas were located in the frontal lobe in 40% of the cases, temporal in 29%, parietal in 14%, and occipital lobe in 3%, with 14% in the deeper structures. The difference in distribution between lobes remained after adjustment for their tissue volume: the tumor:volume ratio was 4.5 for frontal, 4.8 for temporal, and 2.3 for parietal relative to the occipital lobe. The area with the densest occurrence was the anterior subcortical brain. Statistically significant spatial clustering was found in the three-dimensional analysis. No differences in location were found among glioblastoma, diffuse astrocytoma, and oligodendroglioma. Our results demonstrate considerable heterogeneity in the anatomic distribution of gliomas within the brain.
Keywords: brain neoplasms, diagnostic imaging, glioma, incidence
The incidence of gliomas has increased worldwide since the late 1970s.1–5 There are several possible causes for this increase, including improved diagnostic methods, such as modern radiologic imaging, and better access to neurosurgical services.2,3,6–8 Incidental findings of brain neoplasms increased with the introduction of CT and MRI technology in the 1980s.4,8,9 However, it has also been suggested that the overall increase in incidence is leveling off,3 whereas the increasing trend continues in the older age groups.2,3
The anatomic topographic location of a glioma affects treatment options and prognosis.8,10–13 However, few large-scale studies have been published on the detailed anatomic locations of gliomas.12
To date, it has been widely believed that gliomas develop in different lobes with frequencies relative to the volume of glial tissue, reflected in the ratio of gray and white matter. Revealing differences in the anatomic distribution of gliomas may provide further insight into the etiology and pathogenesis of gliomas. It may, for instance, give clues about the role of highly local external exposures such as trauma or electromagnetic radio-frequency fields from mobile phones. Another possibility is that the anatomic structures provide physiologic stimuli to adjacent glial tissue, which affects the susceptibility to malignant transformation. A third possibility is the effect of functional differences among cells and tissues in different areas of the brain on the development of gliomas. Several studies have also shown differences in biologic characteristics (molecular alterations) in subsets of gliomas arising from different locations.14–16
The aim of our study was to describe the anatomic distribution of gliomas, using a representative case series with detailed localization based on radiologic imaging.
Materials and Methods
The cases were identified from the neurosurgery clinics of all five university hospitals (Helsinki, Turku, Tampere, Kuopio, Oulu) in Finland. We retrieved the records of all patients diagnosed with any glioma at these hospitals during the period from November 2000 to September 2002. (In addition to these five hospitals, glioma is treated surgically at only one neurosurgery clinic in Finland; only 10 incident gliomas were diagnosed there during the study period, and these cases were not included in our study.) Two criteria were used to determine eligibility for inclusion in our study. First, all patients were required to be Finnish citizens residing in Finland. Second, those in the study cohort were required to be between the ages of 20 and 69 years. For the latter inclusion criterion, an age limit was imposed because these data were also used in the international INTERPHONE study, which assessed the possible effect of mobile phones on intracranial tumors.17 Patients were approached for recruitment into the study immediately after surgical resection of the neoplasm and pathologic confirmation of the glioma diagnosis (n = 328, 99%). In 1% of cases (n = 3), the diagnosis was based on a radiologic finding (CT and/or MRI) indicating glioma as the tumor type rather than biopsy of a surgical specimen.
The study protocol was approved by the National Ethical Review Board of the Ministry of Health and Social Welfare (ETENE/TUKIJA). The study subjects or their relatives gave written informed consent. Of all the glioma patients diagnosed during the study period, 81% (n = 267) gave their consent for participation. The remaining 61 patients (18%) were not willing to participate for various reasons, mainly poor general condition. Three patients died of their disease soon after diagnosis prior to enrollment and therefore could not participate. For the patients who did not give consent, additional information on the histologic type of the tumor was obtained from the Finnish Cancer Registry, a nationwide, population-based cancer registry with practically complete coverage of cancer cases in Finland.18 However, the specific anatomic location of the tumors was not obtained at the Finnish Cancer Registry.
All cases were classified with a morphologic code according to the third edition of the International Classification of Diseases in Oncology (ICD-O-3).19 For this study, all histologic specimens of the participants were reviewed afterward independently by an experienced neuropathologist (H.H.). Those originally classified by him were reviewed by one of the two other neuropathologists. The gliomas of the patients who did not give consent were reviewed by the study neuropathologist (H.H.). The gliomas were classified into the following groups: pilocytic astrocytoma (grade I; ICD-O-3 code 9421), diffuse astrocytoma (grade II; 9400, 9410, 9411, 9420), anaplastic astrocytoma (grade III; 9401), glioblastoma (grade IV; 9440–9442), all other astrocytomas (9384, 9424), oligodendrocytic gliomas (9450, 9451), mixed gliomas (9382), ependymal tumors (9383, 9391–9394), and choroid plexus tumors (9390).
The tumor location was specified by using radiologic imaging. Thus, all the tumors were assigned a topographic location according to the International Classification of Diseases, version 10 (ICD-10):20 structures of cerebrum other than cortical lobes (ICD-10 code C71.0), cerebrum by lobe (frontal lobe C71.1, temporal lobe C71.2, parietal lobe C71.3, occipital lobe C71.4), ventricles (C71.5), cerebellum (C71.6), brainstem (C71.7), and other anatomic sites (C71.8, C71.9). Gliomas originally assigned to two lobes (with two topographic codes, e.g., frontotemporal) were treated differently from those with some overlap (e.g., predominantly frontal glioma with minor involvement of the temporal lobe). The former were recorded as occurring in two lobes, whereas in the latter case the overlap was ignored and the tumor was classified into one location. If the tumor had some overlap with several areas of the brain, the anatomic site was assigned systematically as the more superficial site versus deeper anatomical structure (e.g., frontal lobe for a tumor close to the sphenoidal wing). In the same fashion, the tumor was given a more anterior location versus posterior (e.g., frontal lobe for frontotemporal cases). A total of 181 gliomas (68%) were found to be located in only one anatomic site, whereas 86 (32%) were overlapping two or more sites.
More detailed analysis of 89 patients 30–69 years of age from Helsinki University Central Hospital was conducted based on radiologic imaging (CT and/or MRI). A neuroradiologist (R.M.) recorded the midpoint of each tumor from the CT/MR images on a 1 × 1– cm grid, separately in three projections (sagittal, coronal, and axial), using software (GridMaster computer program, Vompras, Düsseldorf, Germany) designed for this purpose for the INTERPHONE study.17
The world standard population was used in the age standardization.21 Confidence intervals (CIs) for the incidence rates were calculated under the assumption that the observed numbers of cases follow a Poisson distribution.22 In the analysis of the number of tumors in cerebral lobes, the number of tumors was related to the tissue volume of each lobe. Previously published estimates of the tissue volume in each lobe relative to the occipital lobe were used: frontal lobe, 3; temporal lobe, 2; parietal lobe, 2; occipital lobe, 1.23 The ratio was used to adjust for different sizes of anatomic structures and to estimate incidence corrected for tissue volume.
In the analysis of heterogeneity of tumor distribution by lobe, the statistical significance was evaluated using the chi-square test, with expected frequencies calculated as the mean number of cases per lobe, assuming a uniform distribution across the lobes (in further analysis with adjustment for tissue volume of the lobe).
In the analysis of tumor localization with the three two-dimensional projections, simulation was used to obtain the statistical significance. The midpoint of each tumor was assigned to a square within the projection (i.e., each tumor constituting one observation for the analysis). The observed value of the chi-square test statistic was compared with that obtained by randomly allocating a hypothetical location (square) for 89 tumors in the two-dimensional grid (relevant projection), based on a uniform distribution across the squares. This was repeated 999 times to obtain sufficient precision. The statistical significance was obtained by comparing the observed value of the chi-square statistic with the simulated ones (49 simulations with similar or larger chi-square values corresponding to a significance level of p = 0.05).
Three-dimensional analysis of spatial clustering was evaluated by using a chi-square test. Each glioma was assigned a single midpoint within a cube in the three-dimensional grid. There were four multifocal gliomas with midpoint assigned to the larger tumor. The test statistic was obtained by comparing the observed number of gliomas in the three-dimensional grid with 890 cubes, 1 × 1 × 1 cm in size (some partial), to the expected frequency, obtained by assuming a uniform (random) distribution of tumors across cubes (0.1 tumors per cube). Owing to the small expected frequency, a conventional interpretation based on the chi-square distribution could not be used. Instead, statistical significance was assessed by simulations, randomly assigning a similar number of tumors to the grid. This simulation was repeated 999 times, and the observed chi-square value was compared with those obtained from simulations. A two-sided hypothesis was used, based on the frequency of simulations with similar or higher chi-square statistic (e.g., 49 simulations with chi-square value larger than the observed corresponding to a p value of 0.05). In addition, the mean distance between the midpoints of gliomas was calculated as a summary descriptive measure.
For comparison of the location of histologic types, gliomas were grouped into three morphologic categories: diffuse and anaplastic astrocytomas (grades II and III, n = 19), glioblastomas (n = 34), and a combined group of mixed gliomas and oligodendrogliomas (n = 30).
Results
A total of 331 incident gliomas were diagnosed during our study period. The majority (47%) were glioblastomas. Diffuse astrocytomas accounted for 14%, anaplastic astrocytomas for 9.4%, pilocytic astrocytomas for 5.1%, and all other astrocytomas for 0.6% of the gliomas. Oligodendroglial tumors comprised 11%, mixed gliomas 9.7%, and ependymomas 3.0% of the gliomas (Table 1). In addition, one choroid plexus tumor and one dysembryoplastic neuroepithelial tumor were diagnosed (0.3% each). No substantial differences in histology were found between patients who gave consent to participate and those who did not give consent (p = 0.17). For the former, the average patient age was 49.2 years (median = 51; range = 20–69) and 51.7% were men, whereas for those who did not give consent, the average age was 52.4 years (median = 54.5; range = 26–68) and 54.7% were men.
Table 1.
Number and incidence of gliomas by histologic type
|
No. of Cases (%) |
||||
|---|---|---|---|---|
| Histologic Type | Participants | Nonparticipantsa | Allb | Incidence (/100,000) |
| Glioblastoma | 116 (43) | 37 (61) | 154 (47) | 2.0 |
| Diffuse astrocytoma | 38 (14) | 8 (13) | 46 (14) | 0.7 |
| Anaplastic astrocytoma | 24 (9) | 5 (8) | 31 (9) | 0.5 |
| Pilocytic astrocytoma | 14 (5) | 3 (5) | 17 (5) | 0.3 |
| All other astrocytomas | 2 (< 1) | 0 (0) | 2 (< 1) | 0.03 |
| Oligodendroglioma | 34 (13) | 3 (5) | 37 (11) | 0.5 |
| Mixed glioma | 29 (11) | 3 (5) | 32 (10) | 0.5 |
| Ependymoma | 8 (3) | 2 (3) | 10 (3) | 0.2 |
| Choroid plexus | 1 (< 1) | 0 (0) | 1 (< 1) | 0.01 |
| All other gliomas | 1 (< 1) | 0 (0) | 1 (< 1) | 0.02 |
| Total | 267 | 61 | 331 | 4.7 |
Nonparticipants refers to patients who did not give consent. For these patients, information was obtained from the Finnish Cancer Registry.
Total number (331) includes three persons who died of their disease soon after diagnosis (prior to enrollment) and who are therefore not counted among participants or nonparticipants.
The age-standardized incidence rate was 4.67 per 100,000 person-years (95% CI, 4.2–5.2), with a slightly higher rate for men than women (4.90 compared with 4.47 per 100,000 person-years).
Most of the gliomas were located in the cerebral lobes (86%). Gliomas in the frontal lobe accounted for 40%, temporal lobe for 29%, parietal lobe for 14%, and occipital lobe for 3.0% of the cases. In addition, 6.4% were located primarily in the deep structures of the cerebrum, 2.2% in the ventricles, 1.5% in the cerebellum, and 4.1% in the brainstem.
The crude incidence rate of gliomas (per 100,000) was 1.68 (95% CI, 1.36–2.00) for the frontal lobe, 1.21 (95% CI, 0.94–1.48) for the temporal lobe, 0.58 (95% CI, 0.39–0.77) for the parietal lobe, and 0.13 (95% CI, 0.04–0.21) for the occipital lobe.
Gliomas were located more frequently in the right hemisphere (51%) than in the left (40%). Eleven gliomas were in the center of the brain. Of the 267 gliomas in the study for which the specific anatomic locations of the tumors were available, 13 (4.9%) were bilateral: eight were glioblastomas (ICD-O-3 code 9440), one was a diffuse astrocytoma (9400), two were anaplastic astrocytomas (9401), and two were mixed gliomas (9382). Seven of these bilateral gliomas were located primarily in the frontal lobes. The location of one glioma was unknown.
There were substantial differences in the tumor frequencies between lobes (Table 2). Even after accounting for tissue volume, the frequency was highest for the frontal lobe, followed by the temporal, parietal, and finally the occipital lobe (p < 0.001). The volume-adjusted frequency in the frontal and temporal lobes was roughly four times higher than in the occipital lobe.
Table 2.
Number and frequency of gliomas relative to tissue volume by cerebral lobe
| Location of Glioma | Frequency (No. of Gliomas) | Relative Volumea | Frequency/Volumeb | Frequency: Volume Ratio Relative to Occipital Lobec* |
|---|---|---|---|---|
| Frontal lobe | 107 | 3 | 36 | 4.5 |
| Temporal lobe | 77 | 2 | 39 | 4.8 |
| Parietal lobe | 37 | 2 | 19 | 2.3 |
| Occipital lobe | 8 | 1 | 8 | 1.0 |
Burger et al. 23
Number of cases relative to tissue volume.
Frequency adjusted for tissue volume, with occipital lobe as the reference (assigned a value of 1).
pvalue for difference between lobes < 0.001.
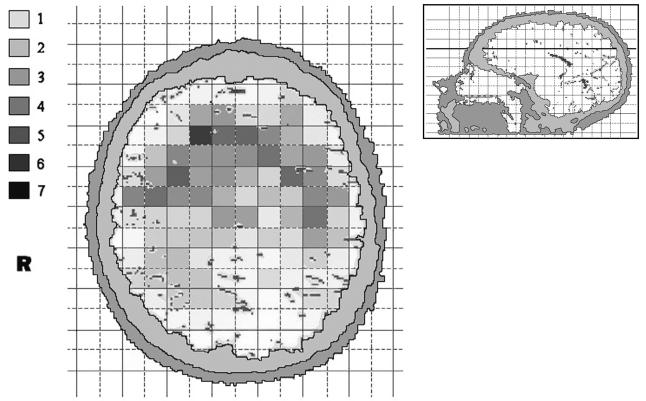
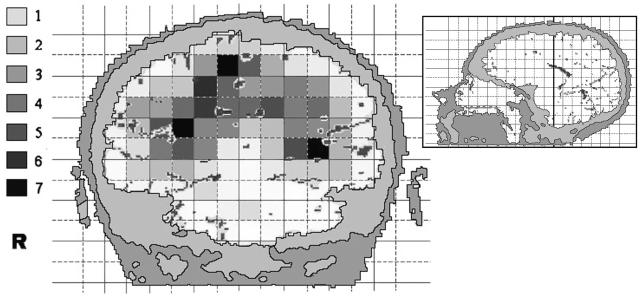
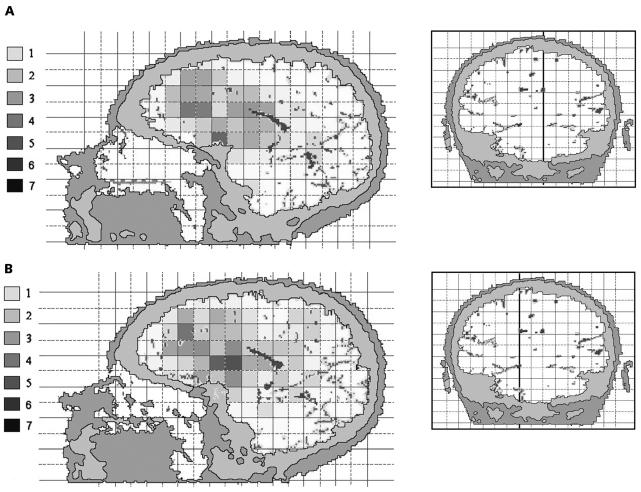
More detailed analyses were performed based on the 89 cases with assessment of CT/MR images. The age distribution of these cases was similar to that for the other cases (average age, 49.2 years compared with 50.3 years, respectively). Also, distribution of histologic type was comparable (47.6% compared with 45.7% of the gliomas were glioblastomas). The tumor localization was characterized using a two-dimensional 1 × 1– cm grid in three projections. In the axial projection, there was some evidence for heterogeneity, that is, uneven distribution across squares with borderline significance (Fig. 1; p = 0.06). The tumors arose most frequently in the anterior subcortical part of the brain. In the coronal projection, there was an inverted U-shape distribution of tumors (Fig. 2; p < 0.001 for difference between squares). In the sagittal projections, the neoplasms were in the anterior areas of the brain and around the sellar region on both the left hemisphere (Fig. 3A; p = 0.007) and right hemisphere (Fig. 3B; p = 0.02).
Fig. 1.
The anatomic site distribution of gliomas in an axial projection of the brain (anterior at top). The colors represent the number of gliomas in each 1 × 1–cm square, with smoothing based on adjacent squares. The inset shows the section plane.
Fig. 2.
The anatomic site distribution of gliomas in a coronal projection of the brain (facing the front). The colors represent the number of gliomas in each 1 × 1–cm square, with smoothing based on adjacent squares. The inset shows the section plane.
Fig. 3.
The anatomic site distribution of gliomas in a sagittal projection of the brain from the left (A) and from the right (B). The colors represent the number of gliomas in each 1 × 1–cm square, with smoothing based on adjacent squares. The inset shows the section plane.
In the three-dimensional analysis, the observed mean distance between the gliomas was 5.17 cm (SEM = 0.09 cm; median = 5.02 cm). There were 11 cubes with two gliomas, providing a chi-square test statistic of 1,021 with 889 degrees of freedom. The frequency of similar or higher chi-square values in the simulations was 7/999, corresponding to a statistical significance of 0.007. The observed mean coordinate was similar to the simulations in the axial and sagittal axes (mean observed and simulated values of 8.6 vs. 8.3 in axial and 6.5 vs. 6.6 in sagittal axis) but slightly different for the coronal axis (10.0 vs. 11.8), indicating a tendency toward upper (superior) parts of the brain.
There were no statistically significant differences in the location of gliomas (based on assessment on the three-dimensional grid) between the three major histologic subtypes. In the three-dimensional analysis, the distances between group-specific midpoints were 1–2 cm (diffuse astrocytoma vs. glioblastoma, 1.2 cm; diffuse astrocytoma vs. oligodendroglioma/mixed glioma, 0.6 cm, glioblastoma vs. oligodendroglioma/mixed glioma, 1.2 cm), indicating closer proximity between the subgroups than with the simulation (2.3 cm for diffuse astrocytoma, 1.4 cm for glioblastoma, and 2.4 cm for oligodendroglioma/mixed glioma).
Discussion
Our results demonstrate considerable differences in distributions of gliomas, with the densest occurrence in the frontal lobe and a higher frequency in the right hemisphere than in the left hemisphere.
The incidence rate (4.67/100,000) of gliomas in our study was comparable with that in a previous report from Finland.24 However, it was relatively high compared with findings in other countries.2,25 The fairly high incidence compared with most other populations is consistent with findings from other Nordic countries.2,4,26 This may be attributable to completeness of registration and rate of histologic confirmation. However, compared with other forms of cancer, brain tumors do not show substantial international variation.27
The incidences of different histologic types of gliomas in this study were comparable with those in previous studies, with astrocytic tumors accounting for more than three quarters of gliomas.25,27 According to the data from the Central Brain Tumor Registry of the United States,25 glioblastomas account for 51%, anaplastic astrocytomas for 8%, and oligodendrogliomas for 10% of all primary brain and CNS gliomas.
The anatomic distribution of gliomas was irregular, with the number of tumors substantially higher for the frontal and temporal lobes than for other lobes, even after adjustment for tissue volume. Furthermore, statistically significant clustering was found in the three-dimensional analysis. No differences were found in distributions of the three major categories of gliomas. These results are consistent with a previous study, which found that 43% of glioblastomas were located in the frontal lobe, 28% in the temporal, 25% in the parietal, and 3% in the occipital lobe.10 The occurrence of bilateral gliomas was toward the frontal lobes, because most gliomas involving both hemispheres are bifrontal.28 Also, the more frequent involvement of the right hemisphere has been reported in a previous study.29 A study of the anatomic distribution of low-grade gliomas found the highest tumor frequency in the secondary functional areas.12 In these studies, however, assessment of tumor location was not as detailed as here.
In more detailed analyses, the tumors were distributed toward frontal subcortical areas. The cortical areas consist of gray material, whereas the subcortical areas contain more glial cells. As gliomas develop from the glial cells, the difference between the cell types in separate areas may explain partly why tumors arise preferably from the subcortical sites. The nonuniform anatomic distribution of gliomas with frontal and temporal predominance may reflect the involvement of developmental, neurochemical, or functional factors in the pathogenesis of gliomas. In one study, allelic loss was most common in oligodendrogliomas located in the same anatomic areas (frontal lobe) where we found the highest tumor frequency.16 It has also been suggested that tumors in different parts of the brain arise from different precursor cells or that differences in the extracellular environment may account for the differences between lobes.14 Furthermore, involvement of structural and functional differences between brain regions, including energy metabolism, architectonic tissue arrangements, and interaction between neuronal and glial cells, has been postulated.12
The topographic location (ICD-10 code) obtained from the medical records and cancer registry for the nationwide material was not always specified with sufficient detail. A simplified classification was used in summarizing the anatomic locations, with preference for lateral (i.e., cortical) and anterior structures. This overestimated slightly the tumor frequency in the superficial and frontal sites. The frequency of gliomas in certain lobes may be slightly overestimated, because some tumors in the unspecified or deep cerebrum were coded into the lobes. For example, the sphenoidal wing can be considered a part of the cerebrum other than the lobes as well as a part of the frontal lobe. However, these issues cannot explain the inhomogeneous distribution of gliomas between cerebral lobes. This limitation in classification did not affect the more detailed analyses. Also, some information was lost in the more detailed analysis, because only the midpoint of the tumor was considered.
The distribution of gliomas by anatomic site differs between adults and children.3 Pilocytic astrocytomas in children occur typically in the cerebellum and brainstem,6,30 whereas in adults diffuse supratentorial astrocytomas predominate.2 Thus, the findings of this study apply to gliomas in adults.
Our study has several advantages. A single neuroradiologist evaluated the location of all gliomas, and histologic subtype was verified by a panel of neuropathologists. We had a representative data set owing to a well-defined source population in addition to a high coverage and a high participation rate. Furthermore, the dropout analysis showed no major differences in tumor types between the patients who gave consent to participate and those who did not give consent. Finally, although the radiologic data for tumors about which detailed location were known came from a single center (Helsinki University Central Hospital), there were no major differences in histologic tumor types or age distribution of patients between Helsinki and the other hospitals.
In conclusion, our findings indicate that gliomas arise mainly from the anterior subcortical structures of the brain, with an excess in the frontal and temporal lobes that is not accounted for by tissue volume alone. These findings, based on a detailed analysis of a large representative case series, consolidate the knowledge regarding localization of gliomas and may provide some insights into the development of gliomas.
Acknowledgments
Financial support was provided by the European Union, the Mobile Manufacturers Forum and GSM Association, the Emil Aaltonen Foundation, the Academy of Finland (grants 48757 and 55163), and the National Technology Agency (TEKES) research program LaVita. The data were collected and the GridMaster software program obtained as part of the INTERPHONE study, coordinated by Dr. Elisabeth Cardis, International Agency for Research on Cancer. We thank Dr. Hannu Kalimo (Department of Pathology, Turku University Hospital) and Dr. Leo Paljärvi (Department of Pathology, Tampere University Hospital) for their contribution to the pathologic classification of the tumors. We also thank Professor Antti Penttinen, University of Jyväskylä, for statistical advice.
References
- 1.Tola MR, Casetta I, Granieri E, et al. Intracranial gliomas in Ferrara, Italy, 1976 to 1991. Acta Neurol Scand. 1994;90:312–317. doi: 10.1111/j.1600-0404.1994.tb02730.x. [DOI] [PubMed] [Google Scholar]
- 2.Inskip P, Linet MS, Heineman EF. Etiology of brain tumors in adults. Epidemiol Rev. 1995;17:382–414. doi: 10.1093/oxfordjournals.epirev.a036200. [DOI] [PubMed] [Google Scholar]
- 3.Legler J, Gloecker Ries LA, Smith MA, et al. Brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382–1390. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 4.Lönn S, Klaeboe L, Mathiesen T, et al. Incidence trends of adult primary intracerebral tumors in four Nordic countries. Int J Cancer. 2004;108:450–455. doi: 10.1002/ijc.11578. [DOI] [PubMed] [Google Scholar]
- 5.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States 1977–2000. Cancer. 2004;101:2293–2299. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 6.Smith MA, Freidlin B, Gloeckler Ries LA, Simon R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90:1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- 7.Sadetzki S, Modan B, Chetrit A, Freedman L. An iatrogenic epidemic of benign meningioma. Am J Epidemiol. 2000;151:266–271. doi: 10.1093/oxfordjournals.aje.a010202. [DOI] [PubMed] [Google Scholar]
- 8.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-Oncology. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preston DL, Ron E, Yonehara S, et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 2002;94:1555–1563. doi: 10.1093/jnci/94.20.1555. [DOI] [PubMed] [Google Scholar]
- 10.Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 11.Jeremic B, Grujicic D, Antunovic V, Djuric L, Stojanovic M, Shibamoto Y. Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol. 1994;21:177–185. doi: 10.1007/BF01052902. [DOI] [PubMed] [Google Scholar]
- 12.Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004;100:2622–2626. doi: 10.1002/cncr.20297. [DOI] [PubMed] [Google Scholar]
- 13.Peters O, Gnekow AK, Rating D, Wolff JEA. Impact of location on outcome in children with low-grade oligodendroglioma. Pediatr Blood Cancer. 2004;43:250–256. doi: 10.1002/pbc.20111. [DOI] [PubMed] [Google Scholar]
- 14.Zlatescu MC, TehraniYazdi A, Sasaki H, et al. Tumor location and growth pattern correlate with genetic signature in oligodendroglial neoplasms. Cancer Res. 2001;61:6713–6715. [PubMed] [Google Scholar]
- 15.Mueller W, Hartmann C, Hoffmann A, et al. Genetic signature of oligoastrocytomas correlates with tumor location and denotes distinct molecular subsets. Am J Pathol. 2002;161:313–319. doi: 10.1016/S0002-9440(10)64183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laigle-Donadey F, Martin-Duverneuil N, Lejeune J, et al. Correlations between molecular profile and radiologic pattern in oligodendroglial tumors. Neurology. 2004;63:2360–2362. doi: 10.1212/01.wnl.0000148642.26985.68. [DOI] [PubMed] [Google Scholar]
- 17.Cardis E, Kilkenny M. International case-control study of adult head and neck tumours: results of the feasibility study. Radiat Prot Dosimetry. 1999;83:179–183. [Google Scholar]
- 18.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Acta Oncol. 1994;33:365–369. doi: 10.3109/02841869409098430. [DOI] [PubMed] [Google Scholar]
- 19.Fritz A, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology, ICD-O. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 20.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, ICD-10. 2. Geneva: World Health Organization; 2005. [Google Scholar]
- 21.Bray F, Guilloux A, Sankila R, Parkin DM. Practical implications of imposing a new world standard population. Cancer Causes Control. 2002;13:175–182. doi: 10.1023/a:1014344519276. [DOI] [PubMed] [Google Scholar]
- 22.Dobson A, Kuulasmaa K, Eberle E, Scherer J. Confidence intervals for weighted sums of Poisson parameters. Stat Med. 1991;10:457–462. doi: 10.1002/sim.4780100317. [DOI] [PubMed] [Google Scholar]
- 23.Burger PC, Sheithauer BW, Vogel FS. Surgical Pathology of the Nervous System and Its Coverings. New York: Churchill Livingstone; 1991. [Google Scholar]
- 24.Kallio M. The incidence of intracranial gliomas in southern Finland. Acta Neurol Scand. 1988;78:480–483. doi: 10.1111/j.1600-0404.1988.tb03691.x. [DOI] [PubMed] [Google Scholar]
- 25.CBTRUS. Statistical Report: Primary Brain Tumors in the United States, 1997–2001. Central Brain Tumor Registry of the United States. 2004 Available at http://www.cbtrus.org/reports//2004-2005/2005report.pdf.
- 26.Kleihues P, Cavenee WK. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: International Agency for Research on Cancer; 2000. [Google Scholar]
- 27.Preston-Martin S, Mack WJ. Neoplasms of the nervous system. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 1996. pp. 1231–1274. [Google Scholar]
- 28.Inskip PD, Tarone RE, Hatch EE, et al. Laterality of brain tumors. Neuroepidemiology. 2003;22:130–138. doi: 10.1159/000068747. [DOI] [PubMed] [Google Scholar]
- 29.Ali Kahn A, O’Brien DF, Kelly P, et al. The anatomical distribution of cerebral gliomas in mobile phone users. Ir Med J. 2003;96:240–242. [PubMed] [Google Scholar]
- 30.Burkhard C, di Patre PL, Schüler G, et al. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003;98:1170–1174. doi: 10.3171/jns.2003.98.6.1170. [DOI] [PubMed] [Google Scholar]