Abstract
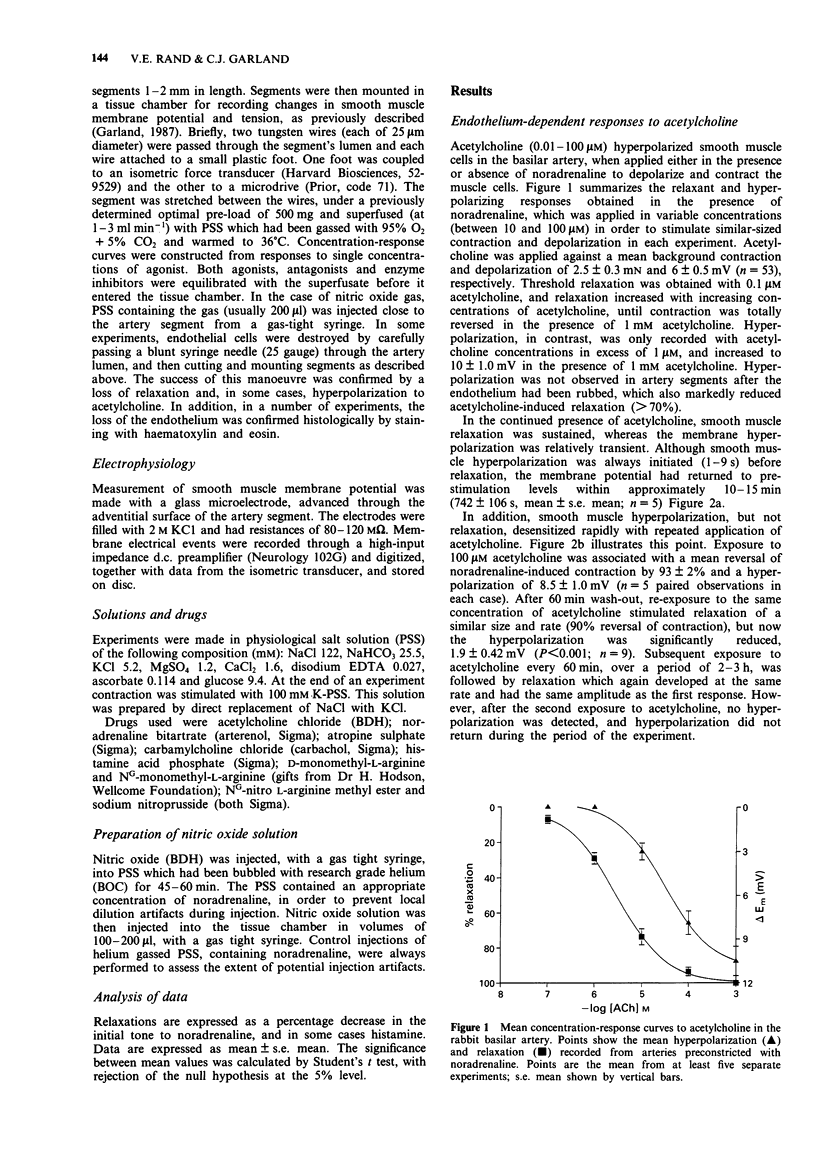
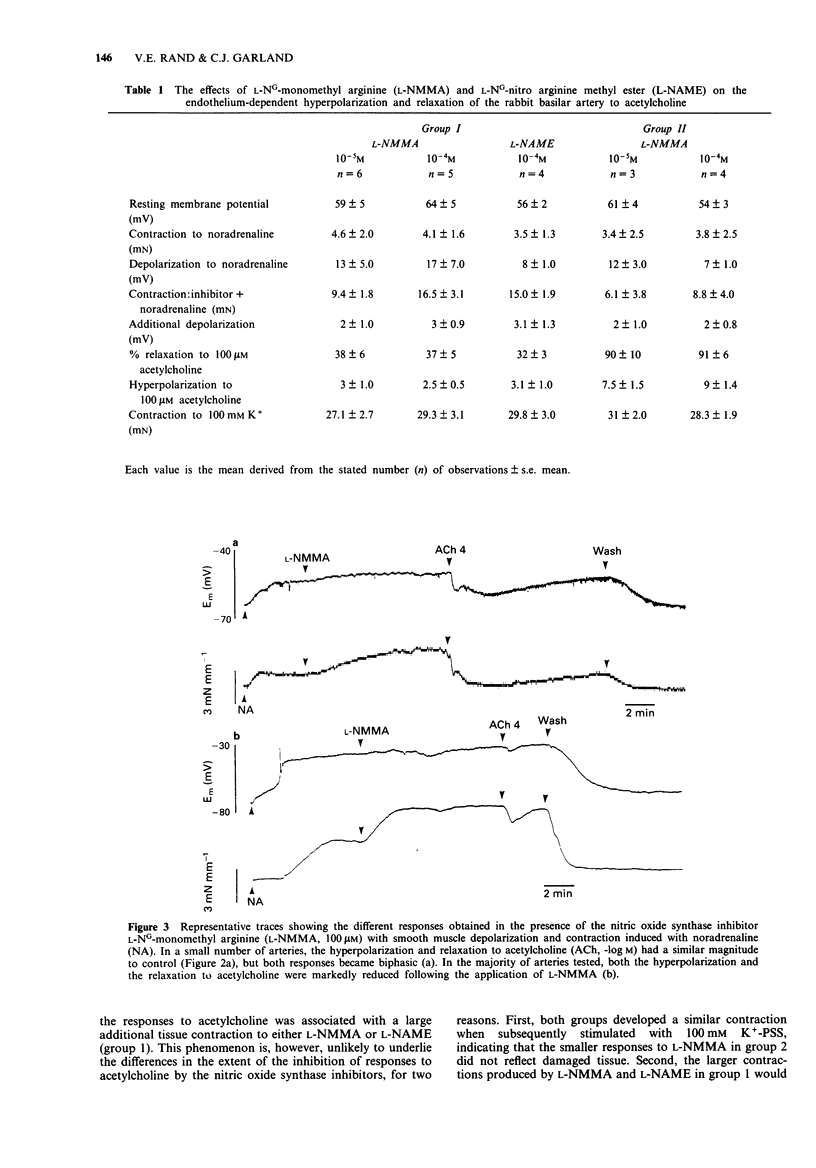
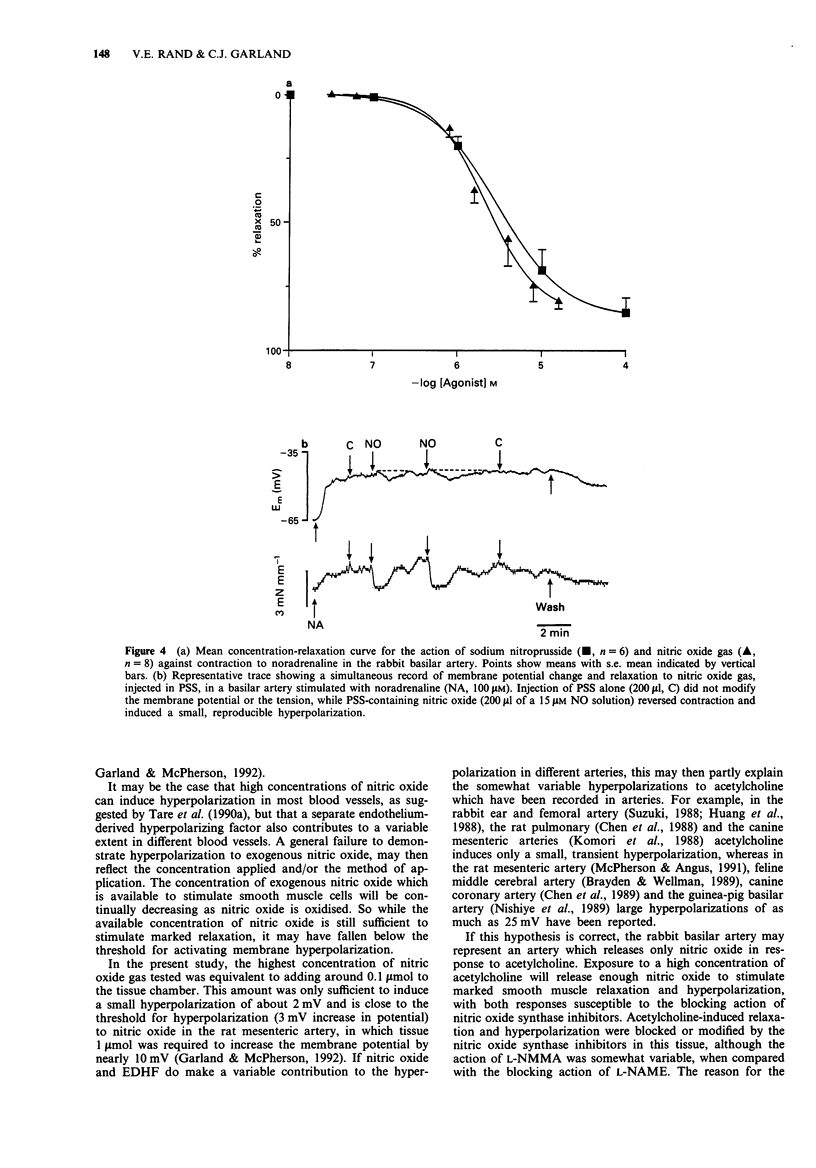
1. Muscarinic stimulation of isolated, preconstricted segments of the basilar artery, with either acetylcholine or carbachol, was followed by endothelium-dependent smooth muscle relaxation and membrane hyperpolarization. 2. Smooth muscle relaxation to acetylcholine was stimulated in the presence of lower concentrations than the associated hyperpolarization (EC50 values 3.2 microM and 31.6 microM, respectively), and was sustained during agonist application, while the hyperpolarization was relatively transient. 3. Repeated exposure to acetylcholine was associated with loss of membrane hyperpolarization, while smooth muscle relaxation was unaltered. Following a second exposure to 100 microM acetylcholine, mean hyperpolarization was markedly depressed from 8.5 to 2 mV, and subsequent exposures failed to induce any hyperpolarization. Relaxations with a similar amplitude and rate of development, were recorded with each subsequent addition of acetylcholine. 4. The competitive substrate inhibitors for nitric oxide synthase, L-NG-monomethyl arginine (100 microM L-NMMA) or L-NG-nitro arginine methyl ester (100 microM L-NAME), modified the form and amplitude of both the relaxation and the hyperpolarization to acetylcholine. In the majority of experiments, both the hyperpolarization and the relaxation were almost totally abolished. 5. Neither nitric oxide, applied directly in physiological salt solution, nor sodium nitroprusside, produced smooth muscle hyperpolarization except in high concentrations. Reproducible, small amplitude (around 2 mV) hyperpolarization followed the application of either NO gas (15 microM) or sodium nitroprusside (100 microM), both of which induced almost maximal smooth muscle relaxation. 6. These data show that muscarinic stimulation of endothelial cells in the rabbit basilar artery is followed by both smooth muscle hyperpolarization and relaxation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C., Schini V. B., Moncada S., Vanhoutte P. M. Stimulation of cyclic GMP production in cultured endothelial cells of the pig by bradykinin, adenosine diphosphate, calcium ionophore A23187 and nitric oxide. Br J Pharmacol. 1990 Sep;101(1):152–156. doi: 10.1111/j.1476-5381.1990.tb12105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray K. M., Newgreen D. T., Small R. C., Southerton J. S., Taylor S. G., Weir S. W., Weston A. H. Evidence that the mechanism of the inhibitory action of pinacidil in rat and guinea-pig smooth muscle differs from that of glyceryl trinitrate. Br J Pharmacol. 1987 Jun;91(2):421–429. doi: 10.1111/j.1476-5381.1987.tb10297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990 Sep;259(3 Pt 2):H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Wellman G. C. Endothelium-dependent dilation of feline cerebral arteries: role of membrane potential and cyclic nucleotides. J Cereb Blood Flow Metab. 1989 Jun;9(3):256–263. doi: 10.1038/jcbfm.1989.42. [DOI] [PubMed] [Google Scholar]
- Chen G., Hashitani H., Suzuki H. Endothelium-dependent relaxation and hyperpolarization of canine coronary artery smooth muscles in relation to the electrogenic Na-K pump. Br J Pharmacol. 1989 Nov;98(3):950–956. doi: 10.1111/j.1476-5381.1989.tb14625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol. 1989 Mar;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Yamamoto Y., Miwa K., Suzuki H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am J Physiol. 1991 Jun;260(6 Pt 2):H1888–H1892. doi: 10.1152/ajpheart.1991.260.6.H1888. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C. J. The role of membrane depolarization in the contractile response of the rabbit basilar artery to 5-hydroxytryptamine. J Physiol. 1987 Nov;392:333–348. doi: 10.1113/jphysiol.1987.sp016783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Busse R., Bassenge E. Endothelium-dependent hyperpolarization of smooth muscle cells in rabbit femoral arteries is not mediated by EDRF (nitric oxide). Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):438–442. doi: 10.1007/BF00172124. [DOI] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- McPherson G. A., Angus J. A. Characterization of responses to cromakalim and pinacidil in smooth and cardiac muscle by use of selective antagonists. Br J Pharmacol. 1990 Jun;100(2):201–206. doi: 10.1111/j.1476-5381.1990.tb15782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson G. A., Angus J. A. Evidence that acetylcholine-mediated hyperpolarization of the rat small mesenteric artery does not involve the K+ channel opened by cromakalim. Br J Pharmacol. 1991 May;103(1):1184–1190. doi: 10.1111/j.1476-5381.1991.tb12321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. The role of hyperpolarization in the relaxation of smooth muscle of monkey coronary artery. J Physiol. 1986 Feb;371:257–265. doi: 10.1113/jphysiol.1986.sp015972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization contributes to endothelium-dependent relaxations to acetylcholine in femoral veins of rats. Am J Physiol. 1991 Oct;261(4 Pt 2):H1034–H1037. doi: 10.1152/ajpheart.1991.261.4.H1034. [DOI] [PubMed] [Google Scholar]
- Nakao K., Okabe K., Kitamura K., Kuriyama H., Weston A. H. Characteristics of cromakalim-induced relaxations in the smooth muscle cells of guinea-pig mesenteric artery and vein. Br J Pharmacol. 1988 Nov;95(3):795–804. doi: 10.1111/j.1476-5381.1988.tb11707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiye E., Nakao K., Itoh T., Kuriyama H. Factors inducing endothelium-dependent relaxation in the guinea-pig basilar artery as estimated from the actions of haemoglobin. Br J Pharmacol. 1989 Mar;96(3):645–655. doi: 10.1111/j.1476-5381.1989.tb11864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Vila E., Macrae I. M., Reid J. L. Differences in inositol phosphate production in blood vessels of normotensive and spontaneously hypertensive rats. Br J Pharmacol. 1991 Oct;104(2):296–300. doi: 10.1111/j.1476-5381.1991.tb12425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


