Abstract
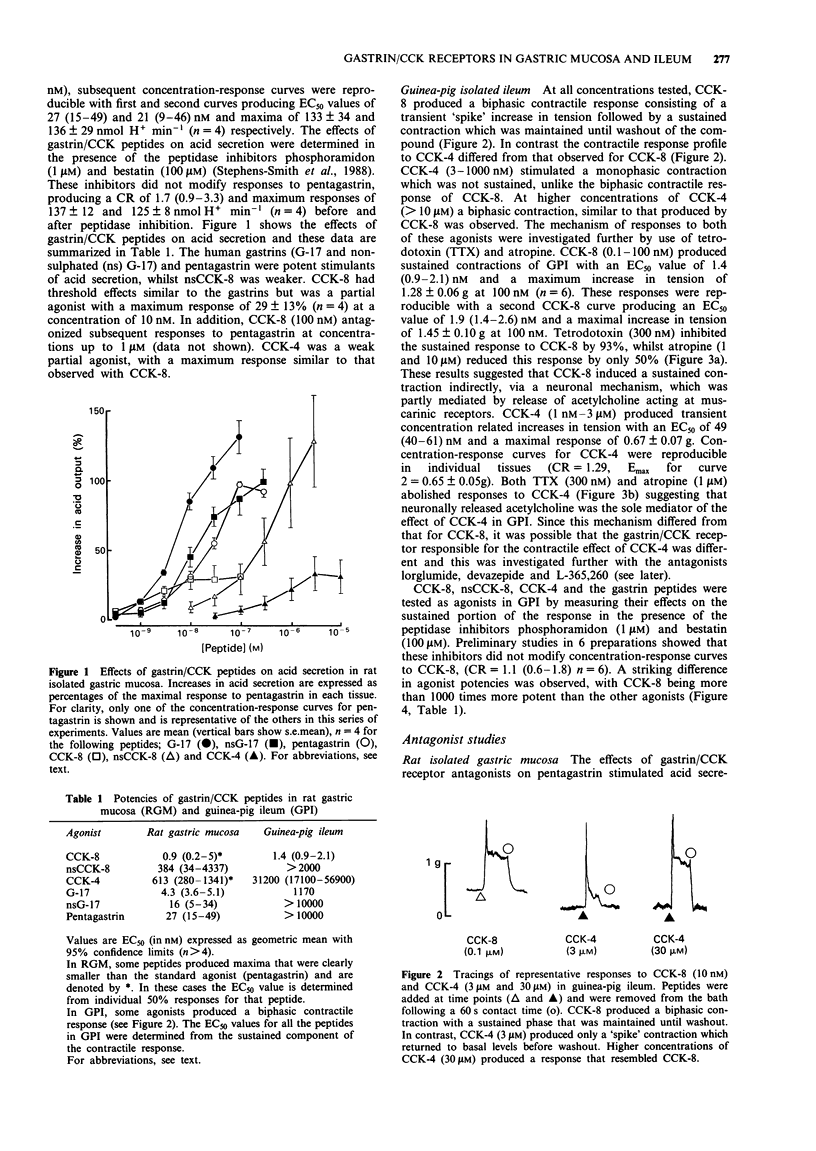
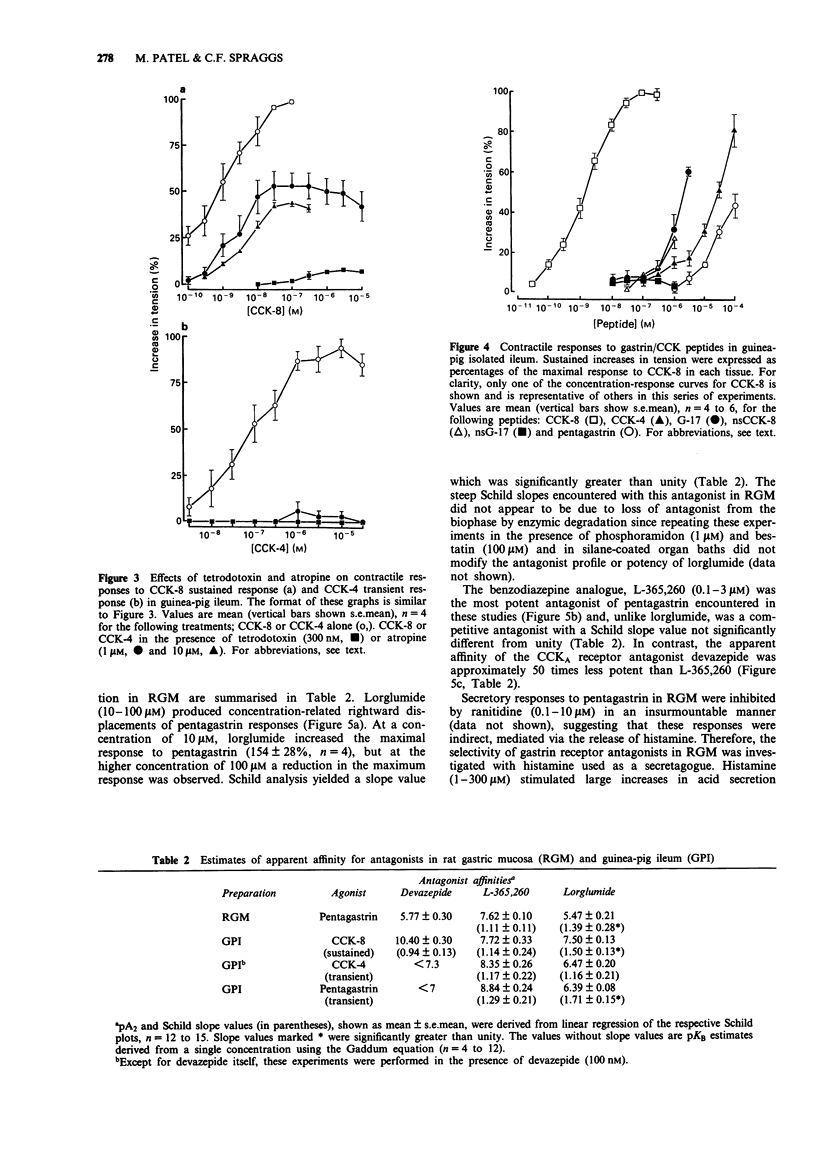
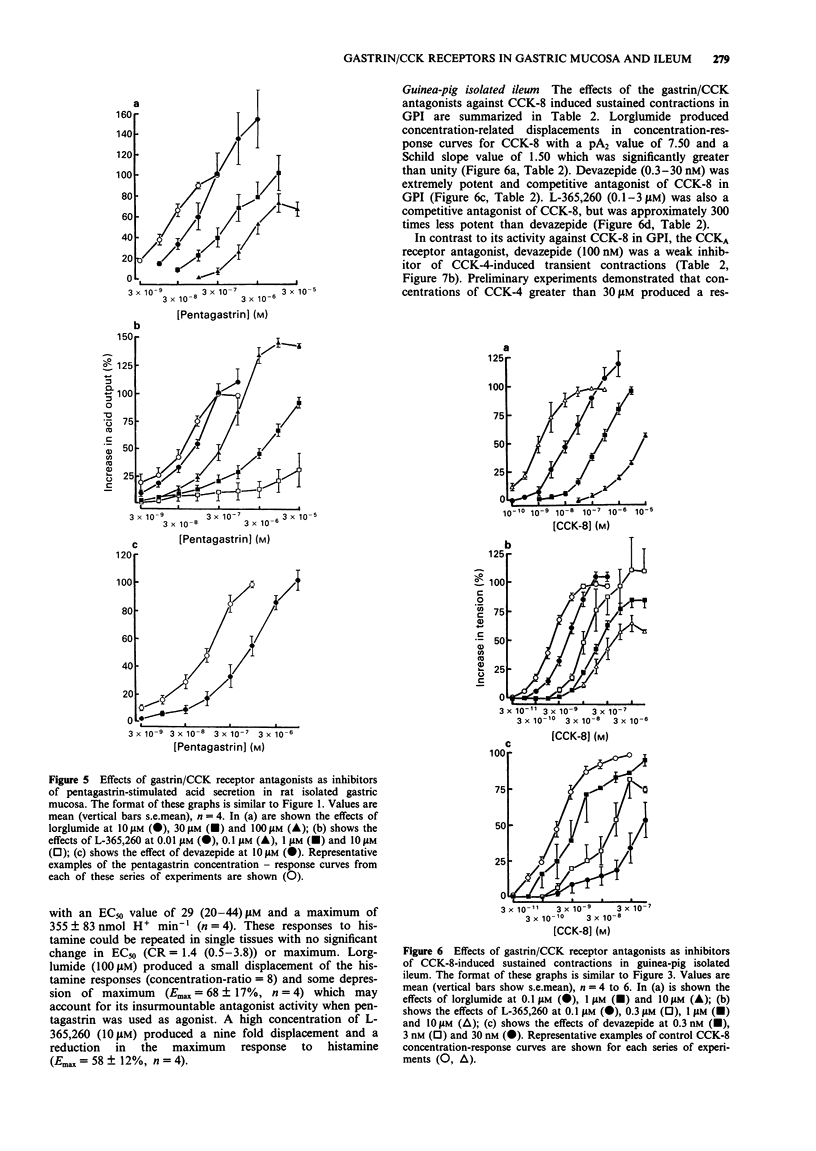
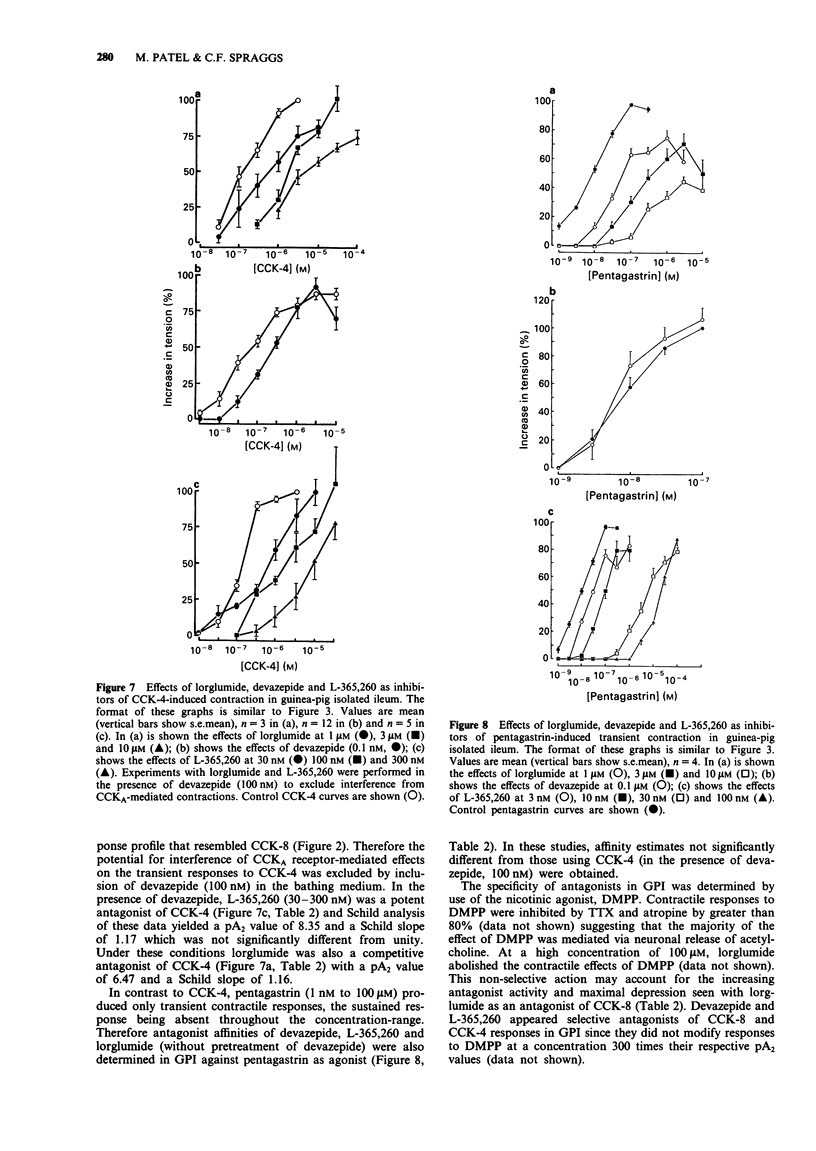
1. The gastrin cholecystokinin (CCK) receptors mediating stimulation of acid secretion in rat isolated gastric mucosa (RGM) and contraction in guinea-pig isolated ileum longitudinal muscle-myenteric plexus (GPI) have been characterized by use of peptide agonists and the non-peptide antagonists, lorglumide, devazepide and L-365,260. 2. In RGM, gastrin peptides (sulphated gastrin heptadecapeptide (G-17), non-sulphated (ns) G-17 and pentagastrin) were potent agonists of acid secretion (EC50 values of 4.3, 16 and 27 nM respectively). Sulphated CCK octapeptide (CCK-8) was also a potent agonist, (EC50 = 0.9 nM), but was less efficacious, producing a lower maximal response. In contrast, in GPI, CCK-8 was a potent full agonist (EC50 = 1.4 nM) and was more than 1000 times more potent than the gastrin peptides in producing a sustained contractile response. 3. In GPI, CCK-8 (0.1 to 100 nM) produced sustained contractile responses, whilst CCK-4 (3 to 1000 nM) produced transient responses. These responses had different sensitivities to atropine (1 microM), suggesting that more than one receptor may mediate contraction in this tissue. 4. In RGM, L-365,260 was the most potent antagonist of pentagastrin-stimulated acid secretion (pA2 = 7.6). This functional affinity estimate was similar to that for L-365,260 as an antagonist of excitatory responses in rat ventromedial hypothalamic slices (Kemp et al., 1989) but differed from binding affinity estimates in guinea-pig cortex and gastric glands (Freidinger, 1989). 5. In GPI, devazepide, L-365,260 and lorglumide yielded different affinity estimates when compared against CCK-8 and CCK-4 or pentagastrin respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitar K. N., Saffouri B., Makhlouf G. M. Cholinergic and peptidergic receptors on isolated human antral smooth muscle cells. Gastroenterology. 1982 May;82(5 Pt 1):832–837. [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P. Pharmacological analysis of the pentagastrin-tiotidine interaction in the mouse isolated stomach. Br J Pharmacol. 1985 Nov;86(3):589–599. doi: 10.1111/j.1476-5381.1985.tb08935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock M. G., DiPardo R. M., Evans B. E., Rittle K. E., Whitter W. L., Veber D. E., Anderson P. S., Freidinger R. M. Benzodiazepine gastrin and brain cholecystokinin receptor ligands: L-365,260. J Med Chem. 1989 Jan;32(1):13–16. doi: 10.1021/jm00121a004. [DOI] [PubMed] [Google Scholar]
- Boden P., Hill R. G. Effects of cholecystokinin and related peptides on neuronal activity in the ventromedial nucleus of the rat hypothalamus. Br J Pharmacol. 1988 May;94(1):246–252. doi: 10.1111/j.1476-5381.1988.tb11521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme G. A., Stutzmann J. M., Blanchard J. C. Excitatory effects of cholecystokinin in rat hippocampus: pharmacological response compatible with 'central'- or B-type CCK receptors. Brain Res. 1988 Jun 7;451(1-2):309–318. doi: 10.1016/0006-8993(88)90776-7. [DOI] [PubMed] [Google Scholar]
- Castro E., Torres M., Miras-Portugal M. T., Gonzalez M. P. Effect of diadenosine polyphosphates on catecholamine secretion from isolated chromaffin cells. Br J Pharmacol. 1990 Jun;100(2):360–364. doi: 10.1111/j.1476-5381.1990.tb15809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Chen T. B. Further evidence that substance P partly mediates the action of cholecystokinin octapeptide (CCK-8) on the guinea pig ileum but not gall bladder: studies with a substance P antagonist. Neurosci Lett. 1984 Apr 20;46(1):71–75. doi: 10.1016/0304-3940(84)90201-5. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Campbell N. J., Shaw T. M., Woodruff G. N. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987 Sep;7(9):2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. C., Zhang L., Chiang H. C., Wank S. A., Maton P. N., Gardner J. D., Jensen R. T. Benzodiazepine analogues L365,260 and L364,718 as gastrin and pancreatic CCK receptor antagonists. Am J Physiol. 1989 Jul;257(1 Pt 1):G169–G174. doi: 10.1152/ajpgi.1989.257.1.G169. [DOI] [PubMed] [Google Scholar]
- Hughes J., Boden P., Costall B., Domeney A., Kelly E., Horwell D. C., Hunter J. C., Pinnock R. D., Woodruff G. N. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6728–6732. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison J. B., Dockray G. J. Evidence that the action of cholecystokinin octapeptide on the guinea pig ileum longitudinal muscle is mediated in part by substance P release from the myenteric plexus. Eur J Pharmacol. 1981 Jan 5;69(1):87–93. doi: 10.1016/0014-2999(81)90605-1. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Snyder S. H. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interactions of COOH-terminal fragments of cholecystokinin with receptors on dispersed acini from guinea pig pancreas. J Biol Chem. 1982 May 25;257(10):5554–5559. [PubMed] [Google Scholar]
- Kondo K., Mitchell J. A., de Nucci G., Vane J. R. Simultaneous measurement of endothelium-derived relaxing factor by bioassay and guanylate cyclase stimulation. Br J Pharmacol. 1989 Oct;98(2):630–636. doi: 10.1111/j.1476-5381.1989.tb12637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. W., Holladay M. W., Barrett R. W., Wolfram C. A., Miller T. R., Witte D., Kerwin J. F., Jr, Wagenaar F., Nadzan A. M. Distinct requirements for activation at CCK-A and CCK-B/gastrin receptors: studies with a C-terminal hydrazide analogue of cholecystokinin tetrapeptide (30-33). Mol Pharmacol. 1989 Dec;36(6):881–886. [PubMed] [Google Scholar]
- Lotti V. J., Chang R. S. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur J Pharmacol. 1989 Mar 21;162(2):273–280. doi: 10.1016/0014-2999(89)90290-2. [DOI] [PubMed] [Google Scholar]
- Lucaites V. L., Mendelsohn L. G., Mason N. R., Cohen M. L. CCK-8, CCK-4 and gastrin-induced contractions in guinea pig ileum: evidence for differential release of acetylcholine and substance P by CCK-A and CCK-B receptors. J Pharmacol Exp Ther. 1991 Feb;256(2):695–703. [PubMed] [Google Scholar]
- Makovec F., Chistè R., Bani M., Pacini M. A., Setnikar I., Rovati L. A. New glutaramic acid derivatives with potent competitive and specific cholecystokinin-antagonistic activity. Arzneimittelforschung. 1985;35(7):1048–1051. [PubMed] [Google Scholar]
- Menozzi D., Gardner J. D., Jensen R. T., Maton P. N. Properties of receptors for gastrin and CCK on gastric smooth muscle cells. Am J Physiol. 1989 Jul;257(1 Pt 1):G73–G79. doi: 10.1152/ajpgi.1989.257.1.G73. [DOI] [PubMed] [Google Scholar]
- Moran T. H., Robinson P. H., Goldrich M. S., McHugh P. R. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986 Jan 1;362(1):175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- Reeves J. J., Stables R. Effects of indomethacin, piroxicam and selected prostanoids on gastric acid secretion by the rat isolated gastric mucosa. Br J Pharmacol. 1985 Nov;86(3):677–684. doi: 10.1111/j.1476-5381.1985.tb08945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F. Four basic characteristics of the gastrin-cholecystokinin system. Am J Physiol. 1981 Apr;240(4):G255–G266. doi: 10.1152/ajpgi.1981.240.4.G255. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Amirian D. A., Thomas L. P., Reedy T. J., Elashoff J. D. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984 May;73(5):1434–1447. doi: 10.1172/JCI111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff G. N., Hughes J. Cholecystokinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:469–501. doi: 10.1146/annurev.pa.31.040191.002345. [DOI] [PubMed] [Google Scholar]
- Yau W. M., Makhlouf G. M., Edwards L. E., Farrar J. T. The action of cholecystokinin and related peptides on guinea pig small intestine. Can J Physiol Pharmacol. 1974 Apr;52(2):289–303. [PubMed] [Google Scholar]



