Abstract
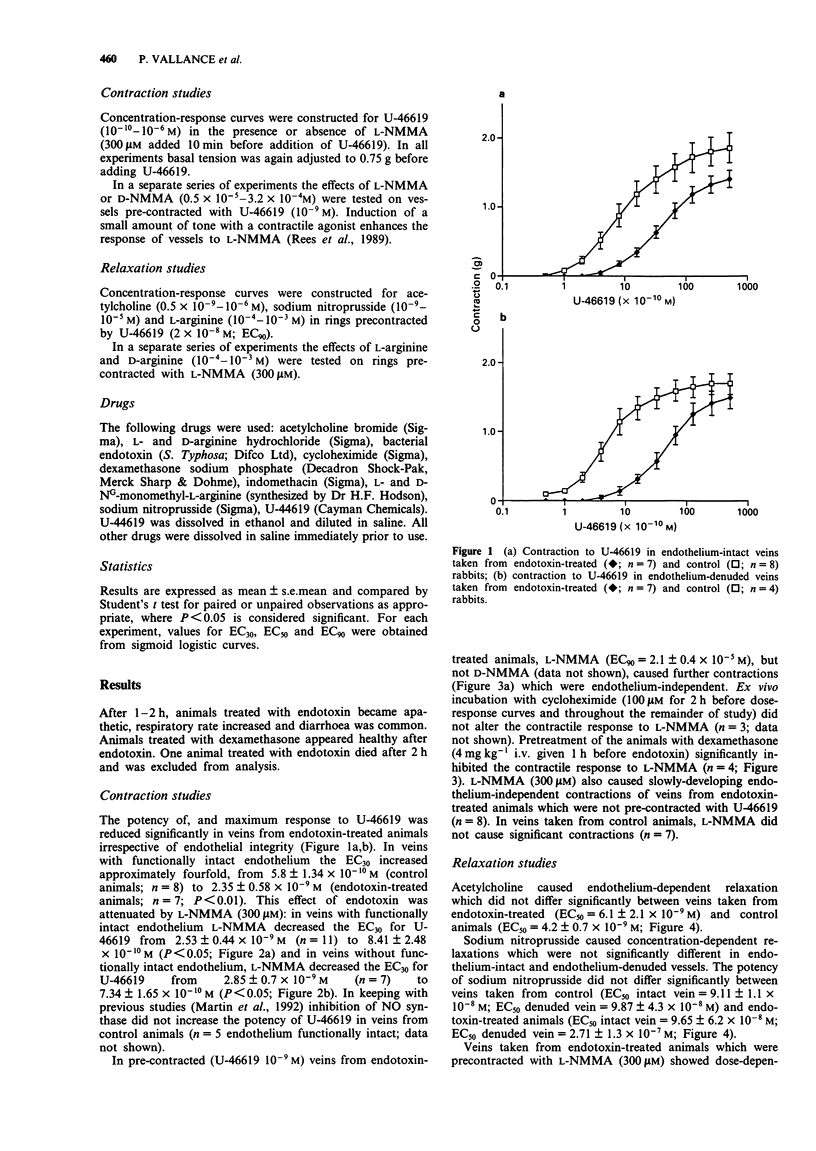
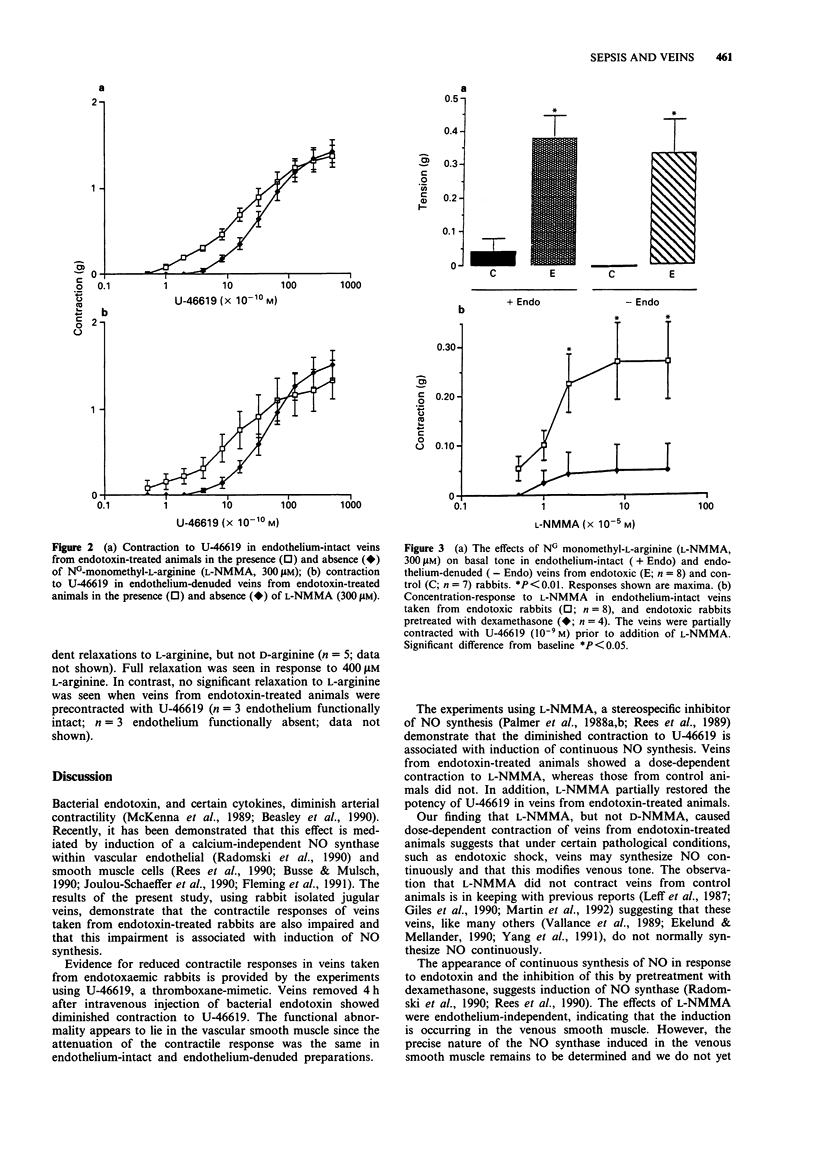
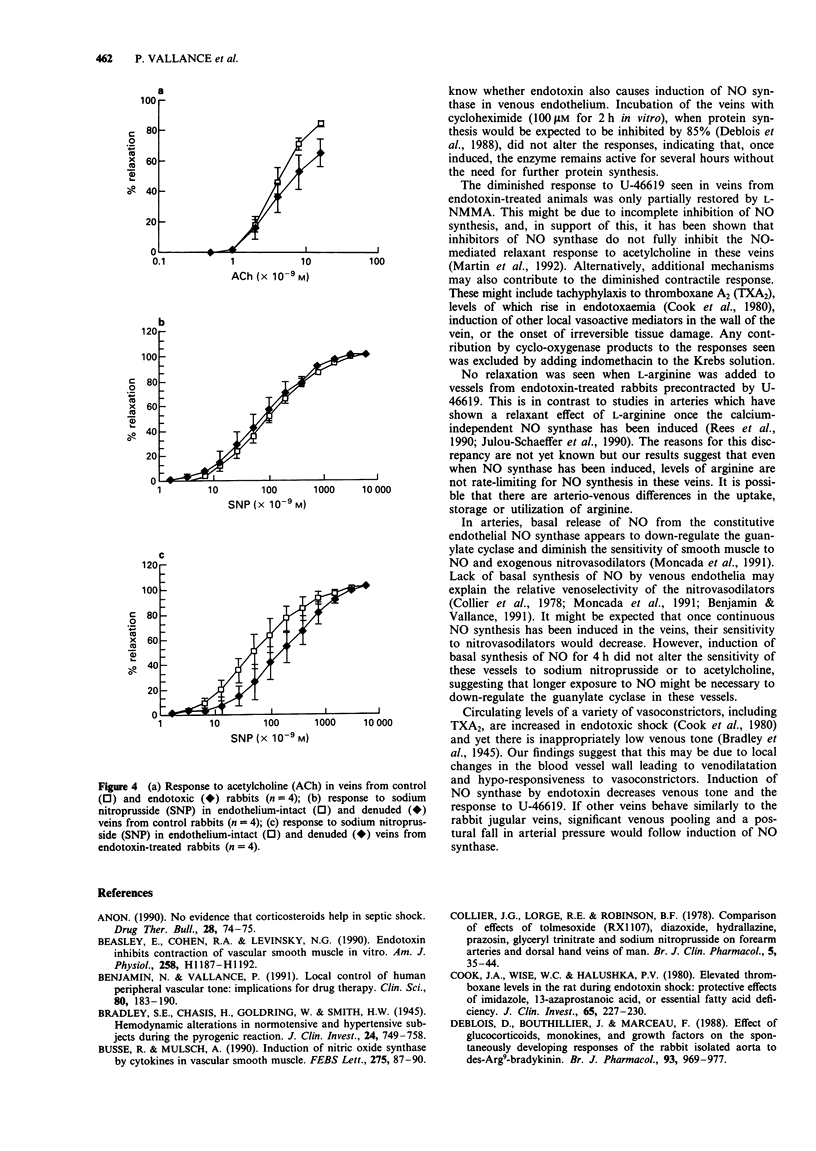
1. Endotoxaemia is characterized by hypotension, peripheral vasodilatation and a reduced response to vasoconstrictors. Clinical studies have indicated that venodilatation contributes to the haemodynamic changes, although there is no direct evidence for abnormal venous reactivity. In the present study, the role of nitric oxide (NO) in modifying the responses of rabbit isolated jugular veins was examined in vitro, 4 h after intravenous injection of endotoxin. 2. Treatment with endotoxin reduced the contractile response to the thromboxane-mimetic, 9,11-dideoxy-11 alpha, 9 alpha-epoxymethano-prostaglandin F2 alpha (U-46619). This affect was endothelium-independent. The response was partially restored by the NO synthase inhibitor. NG-monomethyl-L-arginine (L-NMMA 300 microM). 3. Jugular veins from control animals did not contract to L-NMMA whereas those from endotoxin-treated animals showed concentration-dependent contractions to L-NMMA. The contractions produced by L-NMMA were reversed by L-arginine but not by D-arginine. Treatment of the animals with dexamethasone (4 mg kg-1) 1 h prior to administration of endotoxin significantly attenuated the response to L-NMMA. 4. The response to sodium nitroprusside did not differ significantly between veins from control and endotoxin-treated animals. Endothelial denudation did not alter the sensitivity of the veins to sodium nitroprusside. Acetylcholine produced endothelium-dependent relaxations which were similar in veins from control and endotoxin-treated animals. 5. The results of this study demonstrate that intravenous administration of endotoxin induces hyporesponsiveness to U-46619 in jugular veins. This effect is mediated, at least in part, by the induction of NO synthesis in smooth muscle. The induction is prevented by prior treatment with dexamethasone.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley D., Cohen R. A., Levinsky N. G. Endotoxin inhibits contraction of vascular smooth muscle in vitro. Am J Physiol. 1990 Apr;258(4 Pt 2):H1187–H1192. doi: 10.1152/ajpheart.1990.258.4.H1187. [DOI] [PubMed] [Google Scholar]
- Benjamin N., Vallance P. Local control of human peripheral vascular tone: implications for drug therapy. Clin Sci (Lond) 1991 Mar;80(3):183–190. doi: 10.1042/cs0800183. [DOI] [PubMed] [Google Scholar]
- Bradley S. E., Chasis H., Goldring W., Smith H. W. HEMODYNAMIC ALTERATIONS IN NORMOTENSIVE AND HYPERTENSIVE SUBJECTS DURING THE PYROGENIC REACTION. J Clin Invest. 1945 Sep;24(5):749–758. doi: 10.1172/JCI101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Collier J. G., Lorge R. E., Robinson B. F. Comparison of effects of tolmesoxide (RX71107), diazoxide, hydrallazine, prazosin, glyceryl trinitrate and sodium nitroprusside on forearm arteries and dorsal hand veins of man. Br J Clin Pharmacol. 1978 Jan;5(1):35–44. doi: 10.1111/j.1365-2125.1978.tb01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. A., Wise W. C., Halushka P. V. Elevated thromboxane levels in the rat during endotoxic shock: protective effects of imidazole, 13-azaprostanoic acid, or essential fatty acid deficiency. J Clin Invest. 1980 Jan;65(1):227–230. doi: 10.1172/JCI109655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois D., Bouthillier J., Marceau F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit isolated aorta to des-Arg9-bradykinin. Br J Pharmacol. 1988 Apr;93(4):969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U., Mellander S. Role of endothelium-derived nitric oxide in the regulation of tonus in large-bore arterial resistance vessels, arterioles and veins in cat skeletal muscle. Acta Physiol Scand. 1990 Nov;140(3):301–309. doi: 10.1111/j.1748-1716.1990.tb09004.x. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Schott C., Stoclet J. C. Inducible but not constitutive production of nitric oxide by vascular smooth muscle cells. Eur J Pharmacol. 1991 Aug 6;200(2-3):375–376. doi: 10.1016/0014-2999(91)90602-m. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Leff P., Martin G. R., Morse J. M. Differential classification of vascular smooth muscle and endothelial cell 5-HT receptors by use of tryptamine analogues. Br J Pharmacol. 1987 Jun;91(2):321–331. doi: 10.1111/j.1476-5381.1987.tb10287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna T. M., Lueders J. E., Titius W. A. Monocyte-derived interleukin 1: effects on norepinephrine-stimulated aortic contraction and phosphoinositide turnover. Circ Shock. 1989 Jun;28(2):131–147. [PubMed] [Google Scholar]
- Moncada S., Rees D. D., Schulz R., Palmer R. M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Parker M. M., Natanson C., Suffredini A. F., Danner R. L., Cunnion R. E., Ognibene F. P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990 Aug 1;113(3):227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffredini A. F., Fromm R. E., Parker M. M., Brenner M., Kovacs J. A., Wesley R. A., Parrillo J. E. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989 Aug 3;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Nitric oxide synthesised from L-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc Res. 1989 Dec;23(12):1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., von Segesser L., Bauer E., Stulz P., Turina M., Lüscher T. F. Different activation of the endothelial L-arginine and cyclooxygenase pathway in the human internal mammary artery and saphenous vein. Circ Res. 1991 Jan;68(1):52–60. doi: 10.1161/01.res.68.1.52. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]


