Abstract
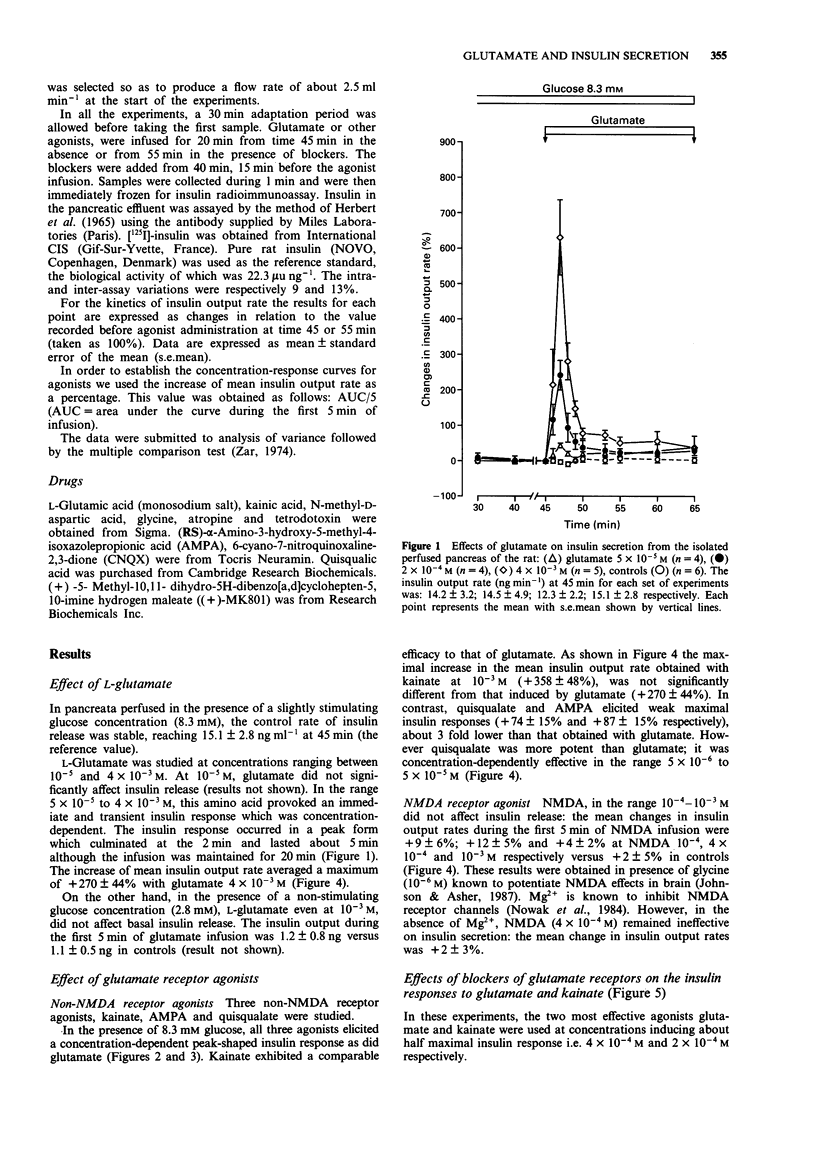
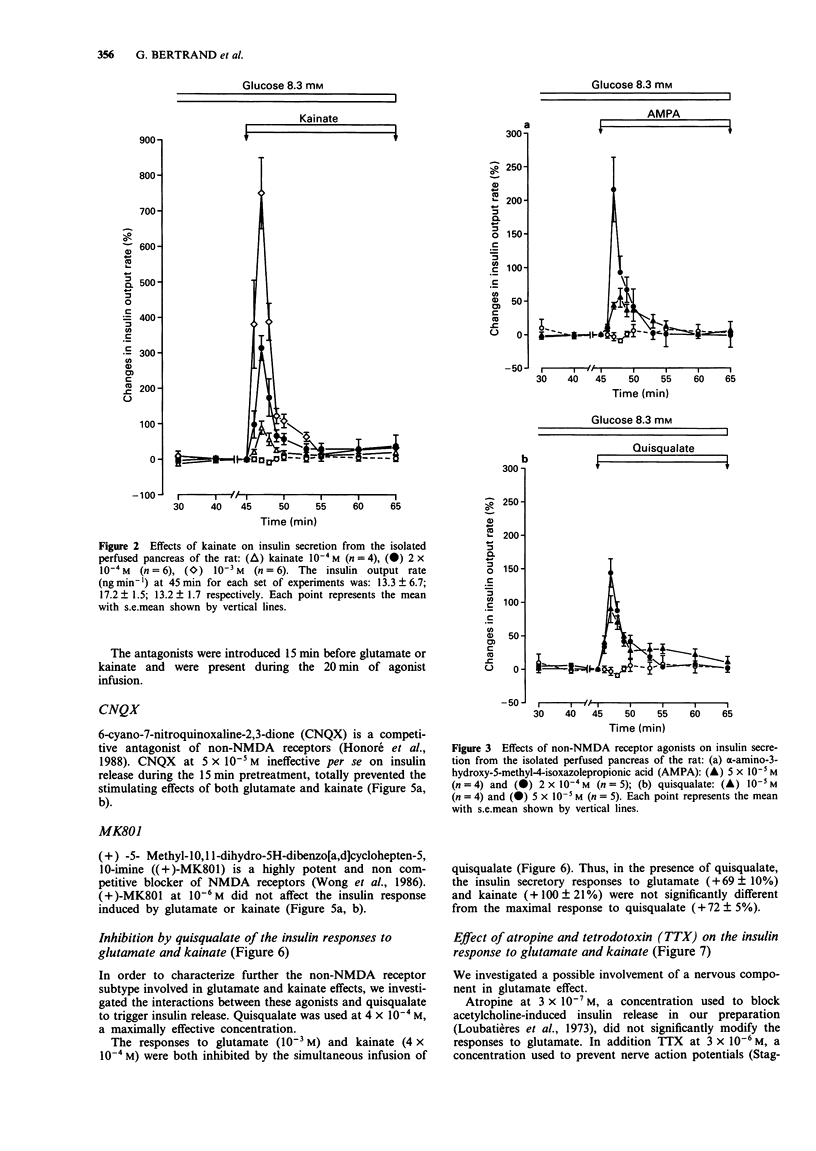
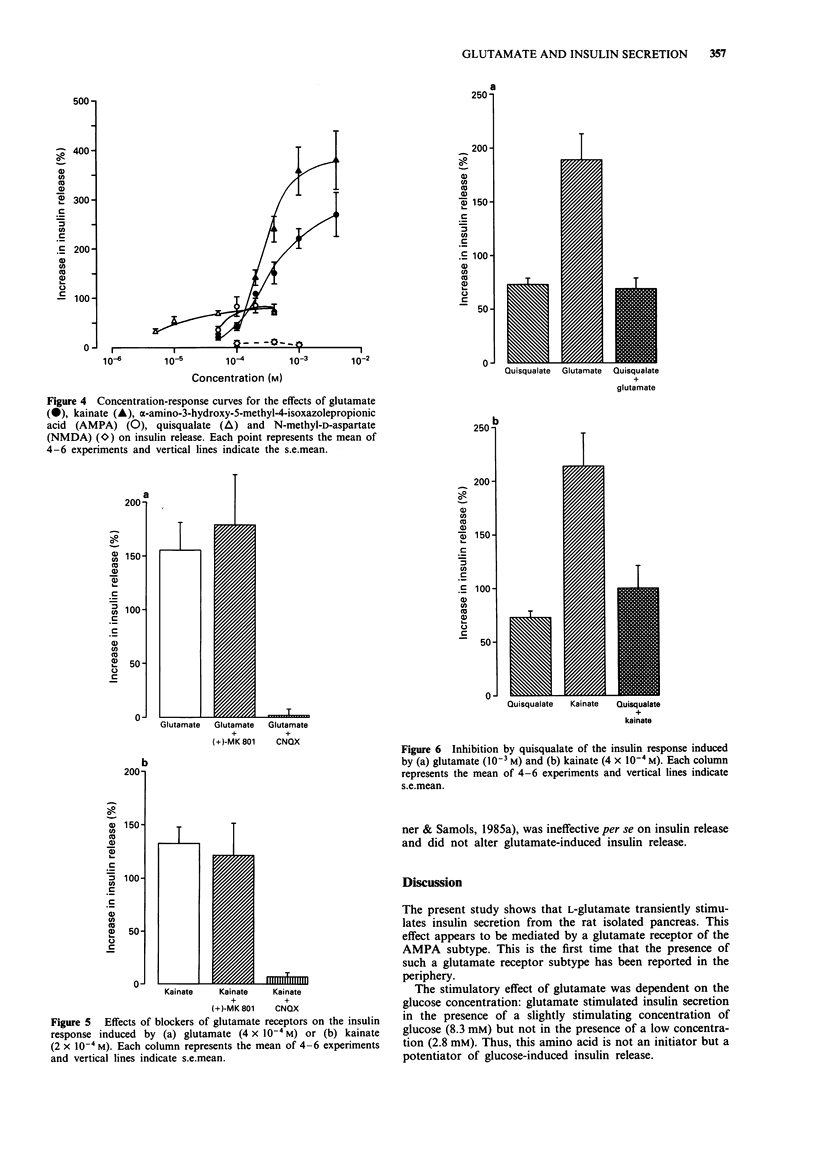
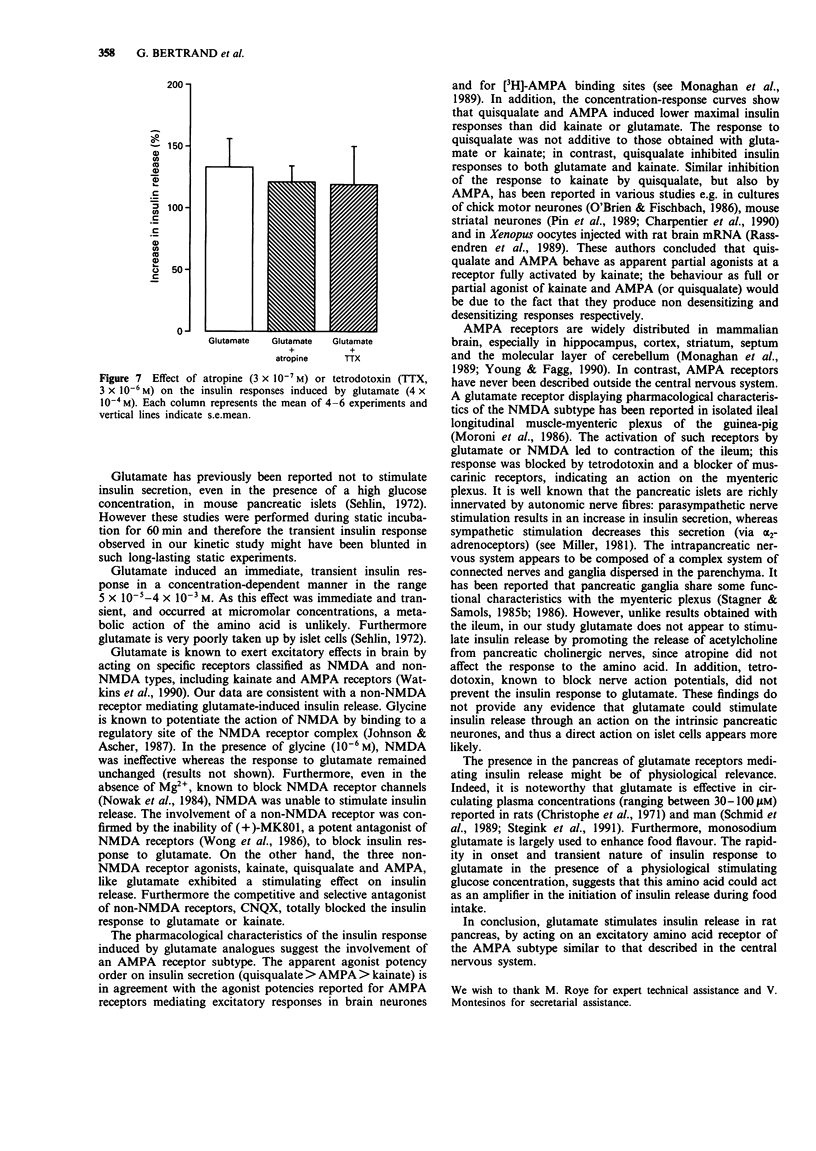
1. The effect of L-glutamate has been studied on insulin secretion by the isolated perfused pancreas of the rat. The glutamate receptor subtype involved has been characterized. 2. In the presence of a slightly stimulating glucose concentration (8.3 mM), L-glutamate (5 x 10(-5)-4 x 10(-3) M) induced an immediate, transient and concentration-dependent insulin response. On the other hand, in the presence of a non stimulating glucose concentration (2.8 mM), L-glutamate (10(-3) M) did not modify the basal insulin secretion. 3. The three non-NMDA receptor agonists, kainate (10(-4)-10(-3) M), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA, 5 x 10(-5)-10(-4) M) and quisqualate (5 x 10(-6)-5 x 10(-5) M) all provoked a transient and concentration-dependent insulin response from pancreas perfused with 8.3 mM glucose. Compared with glutamate, kainate exhibited a similar efficacy, whereas AMPA and quisqualate elicited only a 3 fold lower maximal insulin response. In contrast, NMDA (10(-4)-10(-3) M) was ineffective. 4. An antagonist of non-NMDA receptors, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 5 x 10(-5) M) totally prevented the stimulatory effect of L-glutamate (4 x 10(-4) M) and kainate (2 x 10(-4) M). In contrast, the NMDA receptor antagonist, (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine ((+) MK801) was without effect. 5. The insulin secretory effect of glutamate (4 x 10(-4) M) was not affected by atropine (3 x 10(-7) M) or tetrodotoxin (3 x 10(-6) M). 6. Quisqualate at a high maximally effective concentration (4 x 10(-4) M) inhibited glutamate (10(-3) M) or kainate (4 x 10(-4) M)-induced insulin release. 7. This study shows that L-glutamate stimulates insulin secretion in rat pancreas, by acting on an excitatory amino acid receptor of the AMPA subtype.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand G., Chapal J., Loubatieres-Mariani M. M. Potentiating synergism between adenosine diphosphate or triphosphate and acetylcholine on insulin secretion. Am J Physiol. 1986 Oct;251(4 Pt 1):E416–E421. doi: 10.1152/ajpendo.1986.251.4.E416. [DOI] [PubMed] [Google Scholar]
- Charpentier N., Dumuis A., Sebben M., Bockaert J., Pin J. P. On concanavalin A-treated striatal neurons quisqualate clearly behaves as a partial agonist of a receptor fully activated by kainate. Eur J Pharmacol. 1990 Oct 30;189(4-5):241–251. doi: 10.1016/0922-4106(90)90117-g. [DOI] [PubMed] [Google Scholar]
- Christophe J., Winand J., Kutzner R., Hebbelinck M. Amino acid levels in plasma, liver, muscle, and kidney during and after exercise in fasted and fed rats. Am J Physiol. 1971 Aug;221(2):453–457. doi: 10.1152/ajplegacy.1971.221.2.453. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990 Jul;11(7):290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Loubatières-Mariani M. M., Chapal J., Alric R., Loubatières A. Studies of the cholinergic receptors involved in the secretion of insulin using isolated perfused rat pancreas. Diabetologia. 1973 Dec;9(6):439–446. doi: 10.1007/BF00461685. [DOI] [PubMed] [Google Scholar]
- Loubatières A., Mariani M. M., Ribes G., de Malbosc H., Chapal J. Etude expérimentale d'un nouveau sulfamide hypoglycémiant particulièrement actif, le HB 419 ou glibenclamide. Diabetologia. 1969 Feb;5(1):1–10. doi: 10.1007/BF01212212. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Miller R. E. Pancreatic neuroendocrinology: peripheral neural mechanisms in the regulation of the Islets of Langerhans. Endocr Rev. 1981 Fall;2(4):471–494. doi: 10.1210/edrv-2-4-471. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Moroni F., Luzzi S., Franchi-Micheli S., Zilletti L. The presence of N-methyl-D-aspartate-type receptors for glutamic acid in the guinea pig myenteric plexus. Neurosci Lett. 1986 Jul 11;68(1):57–62. doi: 10.1016/0304-3940(86)90229-6. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Characterization of excitatory amino acid receptors expressed by embryonic chick motoneurons in vitro. J Neurosci. 1986 Nov;6(11):3275–3283. doi: 10.1523/JNEUROSCI.06-11-03275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin J. P., Van Vliet B. J., Bockaert J. Complex interaction between quisqualate and kainate receptors as revealed by measurement of GABA release from striatal neurons in primary culture. Eur J Pharmacol. 1989 Mar 7;172(1):81–91. doi: 10.1016/0922-4106(89)90047-3. [DOI] [PubMed] [Google Scholar]
- Rassendren F. A., Lory P., Pin J. P., Bockaert J., Nargeot J. A specific quisqualate agonist inhibits kainate responses induced in Xenopus oocytes injected with rat brain RNA. Neurosci Lett. 1989 May 8;99(3):333–339. doi: 10.1016/0304-3940(89)90469-2. [DOI] [PubMed] [Google Scholar]
- Reetz A., Solimena M., Matteoli M., Folli F., Takei K., De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991 May;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R., Schusdziarra V., Schulte-Frohlinde E., Maier V., Classen M. Role of amino acids in stimulation of postprandial insulin, glucagon, and pancreatic polypeptide in humans. Pancreas. 1989;4(3):305–314. doi: 10.1097/00006676-198906000-00006. [DOI] [PubMed] [Google Scholar]
- Sehlin J. Uptake and oxidation of glutamic acid in mammalian pancreatic islets. Hormones. 1972;3(3):156–166. doi: 10.1159/000178265. [DOI] [PubMed] [Google Scholar]
- Shannon H. E., Sawyer B. D. Glutamate receptors of the N-methyl-D-aspartate subtype in the myenteric plexus of the guinea pig ileum. J Pharmacol Exp Ther. 1989 Nov;251(2):518–523. [PubMed] [Google Scholar]
- Stagner J. I., Samols E. Modulation of insulin secretion by pancreatic ganglionic nicotinic receptors. Diabetes. 1986 Aug;35(8):849–854. doi: 10.2337/diab.35.8.849. [DOI] [PubMed] [Google Scholar]
- Stagner J. I., Samols E. Perturbation of insulin oscillations by nerve blockade in the in vitro canine pancreas. Am J Physiol. 1985 May;248(5 Pt 1):E516–E521. doi: 10.1152/ajpendo.1985.248.5.E516. [DOI] [PubMed] [Google Scholar]
- Stagner J. I., Samols E. Role of intrapancreatic ganglia in regulation of periodic insular secretions. Am J Physiol. 1985 May;248(5 Pt 1):E522–E530. doi: 10.1152/ajpendo.1985.248.5.E522. [DOI] [PubMed] [Google Scholar]
- Stegink L. D., Filer L. J., Jr, Brummel M. C., Baker G. L., Krause W. L., Bell E. F., Ziegler E. E. Plasma amino acid concentrations and amino acid ratios in normal adults and adults heterozygous for phenylketonuria ingesting a hamburger and milk shake meal. Am J Clin Nutr. 1991 Mar;53(3):670–675. doi: 10.1093/ajcn/53.3.670. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Teitelman G., Lee J. K. Cell lineage analysis of pancreatic islet development: glucagon and insulin cells arise from catecholaminergic precursors present in the pancreatic duct. Dev Biol. 1987 Jun;121(2):454–466. doi: 10.1016/0012-1606(87)90182-5. [DOI] [PubMed] [Google Scholar]
- Terashima T., Katada T., Oinuma M., Inoue Y., Ui M. Endocrine cells in pancreatic islets of Langerhans are immunoreactive to antibody against guanine nucleotide-binding protein (Go) purified from rat brain. Brain Res. 1987 Aug 4;417(1):190–194. doi: 10.1016/0006-8993(87)90199-5. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. B., Fagg G. E. Excitatory amino acid receptors in the brain: membrane binding and receptor autoradiographic approaches. Trends Pharmacol Sci. 1990 Mar;11(3):126–133. doi: 10.1016/0165-6147(90)90199-i. [DOI] [PubMed] [Google Scholar]


