Abstract
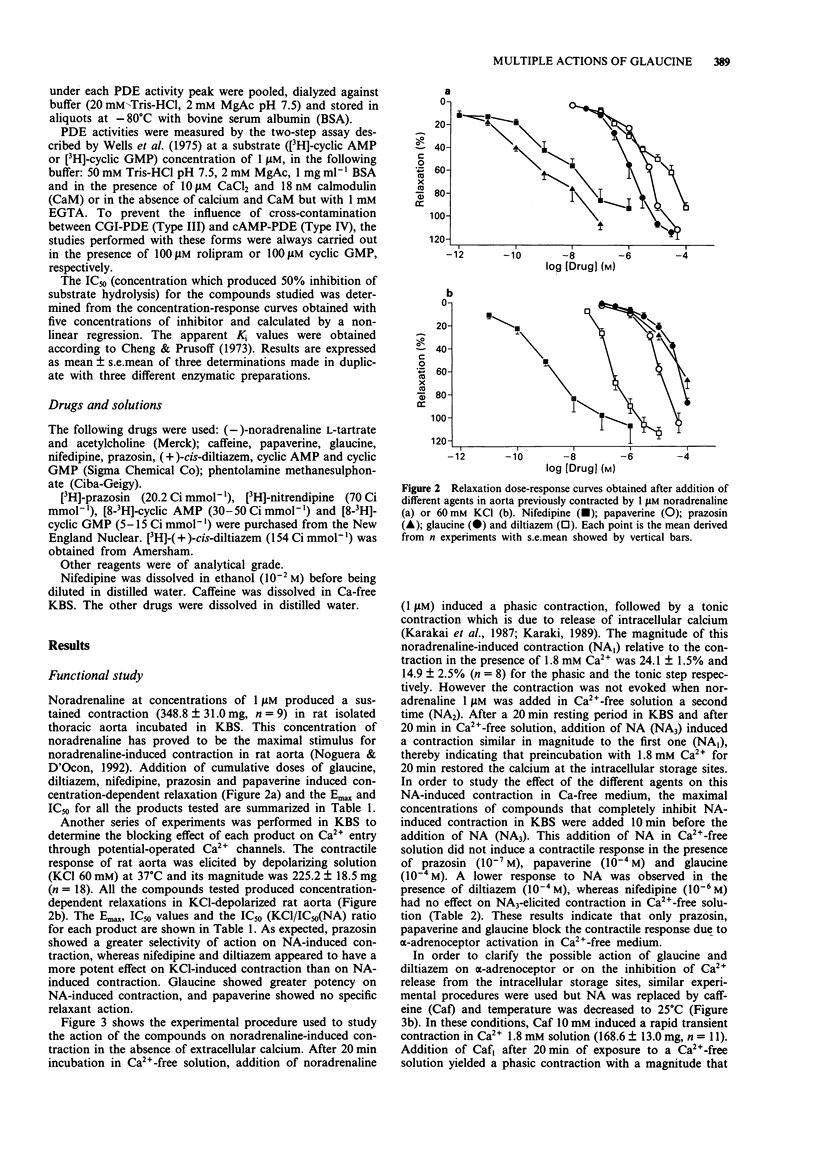
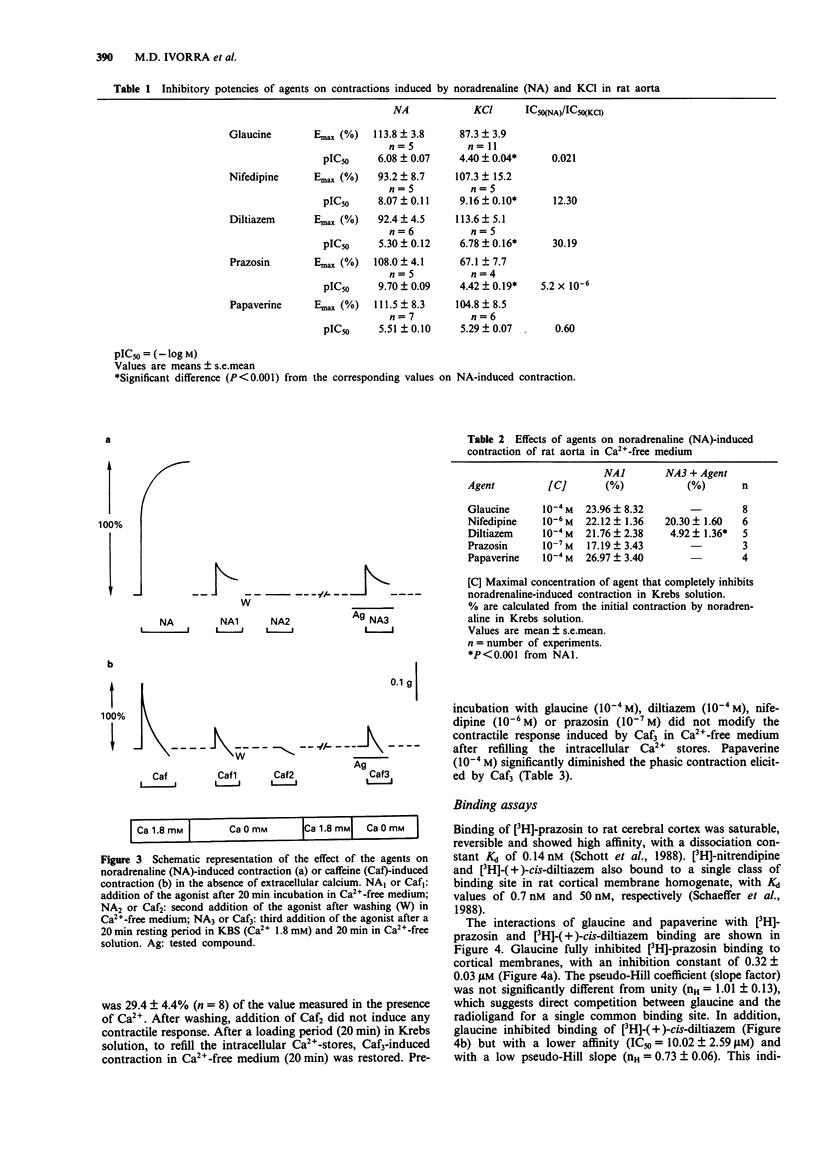
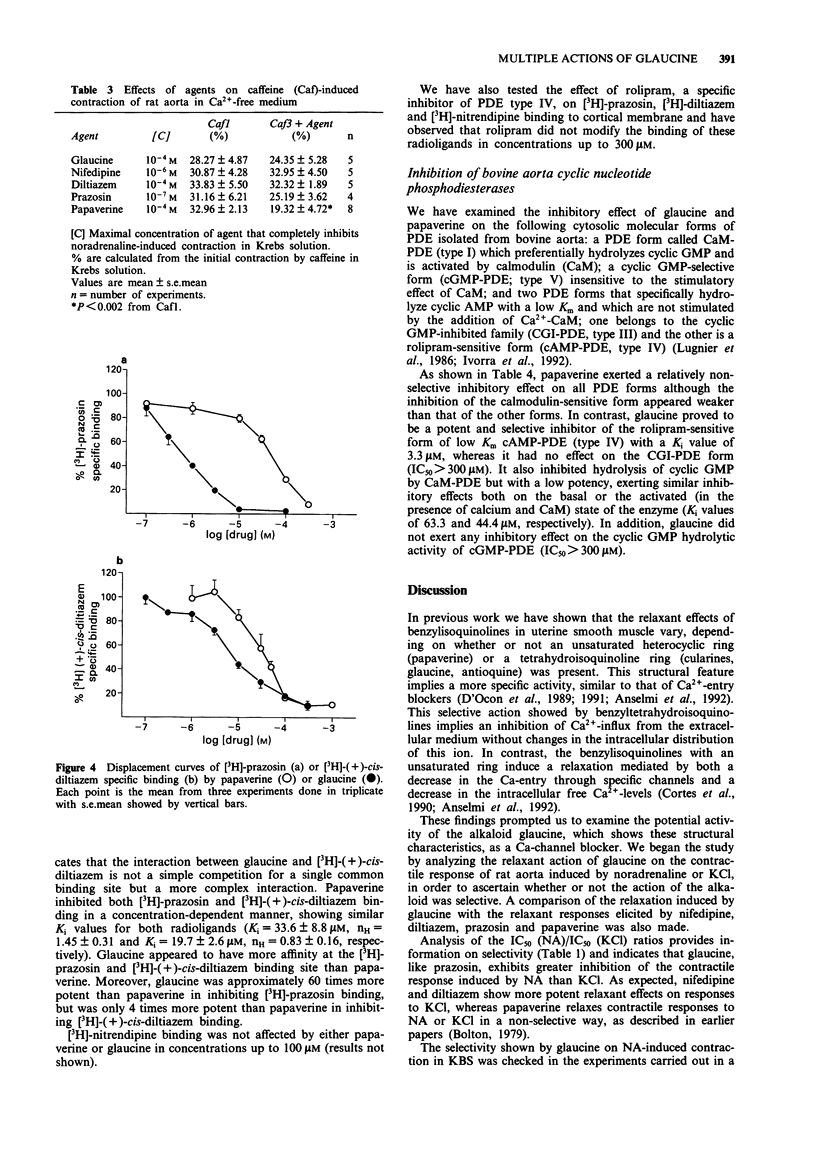
1. In the present study, the properties of glaucine (an aporphine structurally related to papaverine) were compared with those of papaverine, diltiazem, nifedipine and prazosin. The work includes functional studies on rat isolated aorta contracted with noradrenaline, caffeine or KCl, and a determination of the affinity of glaucine at calcium channel binding sites of alpha-adrenoceptors, by use of [3H]-(+)-cis-diltiazem, [3H]-nitrendipine and [3H]-prazosin binding to cerebral cortical membranes. The effects of glaucine on the different molecular forms of cyclic nucleotide phosphodiesterases (PDE) isolated from bovine aorta were also determined. 2. Contraction evoked by noradrenaline (1 microM) or depolarizing solution (60 mM KCl) were inhibited in a concentration-dependent manner by all the compounds tested. As expected, prazosin showed a greater selectivity of action on NA-induced contraction, whereas nifedipine and diltiazem appeared more potent on KCl-induced contraction. Glaucine had a greater potency on the contraction elicited by noradrenaline whereas papaverine acted non specifically. 3. In Ca(2+)-free solution, prazosin (0.1 microM) and glaucine (0.1 mM) inhibited the contraction evoked by NA; diltiazem (0.1 mM) diminished this contraction whereas nifedipine (1 microM) had no effect. Preincubation of tissues with glaucine, diltiazem, nifedipine and prazosin did not modify the contractile response induced by caffeine. In contrast, papaverine (0.1 mM) significantly inhibited the contractions evoked by NA or caffeine in Ca(2+)-free medium. 4. Glaucine and papaverine show affinity at the [3H]-prazosin binding site and at the benzothiazepine binding site of the Ca(2+)-channel receptor complex, but have no effect at the dihydropyridine binding site in rat cerebral cortex.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn H. S., Crim W., Romano M., Sybertz E., Pitts B. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem Pharmacol. 1989 Oct 1;38(19):3331–3339. doi: 10.1016/0006-2952(89)90631-x. [DOI] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Bozarth J. M., Timmermans P. B. Relationship between phosphatidylinositol turnover and Ca++ mobilization induced by alpha-1 adrenoceptor stimulation in the rat aorta. J Pharmacol Exp Ther. 1987 Jan;240(1):123–127. [PubMed] [Google Scholar]
- Cortes D., Torrero M. Y., Pilar D'Ocon M., Luz Candenas M., Cavé A., Hadi A. H. Norstephalagine et atherospermidine, deux aporphines d'Artabotrys maingayi relaxantes du muscle lisse. J Nat Prod. 1990 Mar-Apr;53(2):503–508. doi: 10.1021/np50068a039. [DOI] [PubMed] [Google Scholar]
- Cumiskey W. R., Feigenson M. E. Spasmolytic activity of cinnamedrine and papaverine in isolated rat uterine muscle. Arch Int Pharmacodyn Ther. 1983 May;263(1):113–119. [PubMed] [Google Scholar]
- D'Ocon M. P., Candenas M. L., Anselmi E., Zafra-Polo M. C., Cortes D. Antioquine: a new bisbenzylisoquinoleine alkaloid with calcium antagonist activity. Arch Int Pharmacodyn Ther. 1989 Jan-Feb;297:205–216. [PubMed] [Google Scholar]
- D'Ocon P., Blasco R., Candenas L., Ivorra D., López S., Villaverde C., Castedo L., Cortes D. Inhibition of calcium entry induced by cularines and isocrasifoline in uterine smooth muscle. Eur J Pharmacol. 1991 Apr 17;196(2):183–187. doi: 10.1016/0014-2999(91)90426-q. [DOI] [PubMed] [Google Scholar]
- Daly C. J., Dunn W. R., McGrath J. C., Miller D. J., Wilson V. G. An examination of the sources of calcium for contractions mediated by postjunctional alpha 1- and alpha 2-adrenoceptors in several blood vessels isolated from the rabbit. Br J Pharmacol. 1990 Feb;99(2):253–260. doi: 10.1111/j.1476-5381.1990.tb14690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Ahn H. Y., Urakawa N. Caffeine-induced contraction in vascular smooth muscle. Arch Int Pharmacodyn Ther. 1987 Jan;285(1):60–71. [PubMed] [Google Scholar]
- Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989 Aug;10(8):320–325. doi: 10.1016/0165-6147(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Kauffman R. F., Schenck K. W., Utterback B. G., Crowe V. G., Cohen M. L. In vitro vascular relaxation by new inotropic agents: relationship to phosphodiesterase inhibition and cyclic nucleotides. J Pharmacol Exp Ther. 1987 Sep;242(3):864–872. [PubMed] [Google Scholar]
- King V. F., Garcia M. L., Himmel D., Reuben J. P., Lam Y. K., Pan J. X., Han G. Q., Kaczorowski G. J. Interaction of tetrandrine with slowly inactivating calcium channels. Characterization of calcium channel modulation by an alkaloid of Chinese medicinal herb origin. J Biol Chem. 1988 Feb 15;263(5):2238–2244. [PubMed] [Google Scholar]
- Kithas P. A., Artman M., Thompson W. J., Strada S. J. Subcellular distribution of high-affinity type IV cyclic AMP phosphodiesterase activity in rabbit ventricular myocardium: relations to the effects of cardiotonic drugs. Circ Res. 1988 Apr;62(4):782–789. doi: 10.1161/01.res.62.4.782. [DOI] [PubMed] [Google Scholar]
- Komas N., Lugnier C., Le Bec A., Serradeil-Le Gal C., Barthélémy G., Stoclet J. C. Differential sensitivity to cardiotonic drugs of cyclic AMP phosphodiesterases isolated from canine ventricular and sinoatrial-enriched tissues. J Cardiovasc Pharmacol. 1989 Aug;14(2):213–220. doi: 10.1097/00005344-198908000-00005. [DOI] [PubMed] [Google Scholar]
- Komas N., Lugnier C., Stoclet J. C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br J Pharmacol. 1991 Oct;104(2):495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukovetz W. R., Pöch G. Inhibition of cyclic-3',5'-nucleotide-phosphodiesterase as a possible mode of action of papaverine and similarly acting drugs. Naunyn Schmiedebergs Arch Pharmakol. 1970;267(2):189–194. doi: 10.1007/BF00999402. [DOI] [PubMed] [Google Scholar]
- Lacroix P., Linee P., Forest M. C. Diproteverine (BRL 40015): a new type of calcium antagonist with potential antianginal properties. Eur J Pharmacol. 1991 Jan 17;192(3):317–327. doi: 10.1016/0014-2999(91)90220-k. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Bertrand Y., Stoclet J. C. Cyclic nucleotide phosphodiesterase inhibition and vascular smooth muscle relaxation. Eur J Pharmacol. 1972 Jul;19(1):134–136. doi: 10.1016/0014-2999(72)90090-8. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Nemoz G., Prigent A. F., Moueqqit M., Fougier S., Macovschi O., Pacheco H. Selective inhibition of one of the cyclic AMP phosphodiesterases from rat brain by the neurotropic compound rolipram. Biochem Pharmacol. 1985 Aug 15;34(16):2997–3000. doi: 10.1016/0006-2952(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Noguera M. A., D'Ocon M. P. Different and common intracellular calcium-stores mobilized by noradrenaline and caffeine in vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):333–341. doi: 10.1007/BF00168695. [DOI] [PubMed] [Google Scholar]
- Reeves M. L., Leigh B. K., England P. J. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987 Jan 15;241(2):535–541. doi: 10.1042/bj2410535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D., Roggenbach W., Schmidt U., Schümann H. J. Effect of papaverine on the frequency-force relationship in guinea-pig left atria. Eur J Pharmacol. 1977 Jan 21;41(2):123–132. doi: 10.1016/0014-2999(77)90201-1. [DOI] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Multiple effects of caffeine on contraction and cytosolic free Ca2+ levels in vascular smooth muscle of rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):443–448. doi: 10.1007/BF00172125. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Lugnier C., Stoclet J. C. Interaction of calmodulin and calcium antagonists with [3H]diltiazem and [3H]nitrendipine binding sites. J Cardiovasc Pharmacol. 1988;12 (Suppl 4):S102–S103. doi: 10.1097/00005344-198806124-00021. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Brooker G., Sperelakis N. Papaverine blockade of an inward slow Ca2+ current in guinea pig heart. J Mol Cell Cardiol. 1975 Nov;7(11):867–876. doi: 10.1016/0022-2828(75)90137-6. [DOI] [PubMed] [Google Scholar]
- Schott C., Tetsi L., Heitz C., Stambach J. F., Jung L., Stoclet J. C. Stereoselective blockade of alpha-adrenoceptors by berbine derivatives. Arzneimittelforschung. 1988 Nov;38(11):1567–1571. [PubMed] [Google Scholar]
- Schultz J. E., Schmidt B. H. Rolipram, a stereospecific inhibitor of calmodulin-independent phosphodiesterase, causes beta-adrenoceptor subsensitivity in rat cerebral cortex. Naunyn Schmiedebergs Arch Pharmacol. 1986 May;333(1):23–30. doi: 10.1007/BF00569655. [DOI] [PubMed] [Google Scholar]
- Strada S. J., Martin M. W., Thompson W. J. General properties of multiple molecular forms of cyclic nucleotide phosphodiesterase in the nervous system. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:13–29. [PubMed] [Google Scholar]
- Sutor B., Alzheimer C., Ameri A., ten Bruggencate G. The low KM-phosphodiesterase inhibitor denbufylline enhances neuronal excitability in guinea pig hippocampus in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1990 Sep;342(3):349–356. doi: 10.1007/BF00169448. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Langs D. A., Janis R. A. Ca2+ channel ligands: structure-function relationships of the 1,4-dihydropyridines. Med Res Rev. 1989 Apr-Jun;9(2):123–180. doi: 10.1002/med.2610090203. [DOI] [PubMed] [Google Scholar]
- Van Inwegen R. G., Salaman P., St Georgiev V., Weinryb I. Dihydro- and tetrahydroisoquinolines as inhibitors of cyclic nucleotide phosphodiesterases from dog heart. Structure-activity relationships. Biochem Pharmacol. 1979 Apr 15;28(8):1307–1312. doi: 10.1016/0006-2952(79)90430-1. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Cain M. H., Bristol J. A. A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem. 1985 May;28(5):537–545. doi: 10.1021/jm50001a001. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Kobylarz-Singer D. C., Steffen R. P., Kaplan H. R. Subclasses of cyclic AMP-specific phosphodiesterase in left ventricular muscle and their involvement in regulating myocardial contractility. Circ Res. 1987 Oct;61(4):539–547. doi: 10.1161/01.res.61.4.539. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Baird C. E., Hardman Y. J., Wu J. G. Cyclic nucleotide phosphodiesterase activities of pig coronary arteries. Biochim Biophys Acta. 1975 Apr 19;384(2):430–442. doi: 10.1016/0005-2744(75)90044-3. [DOI] [PubMed] [Google Scholar]


