Abstract
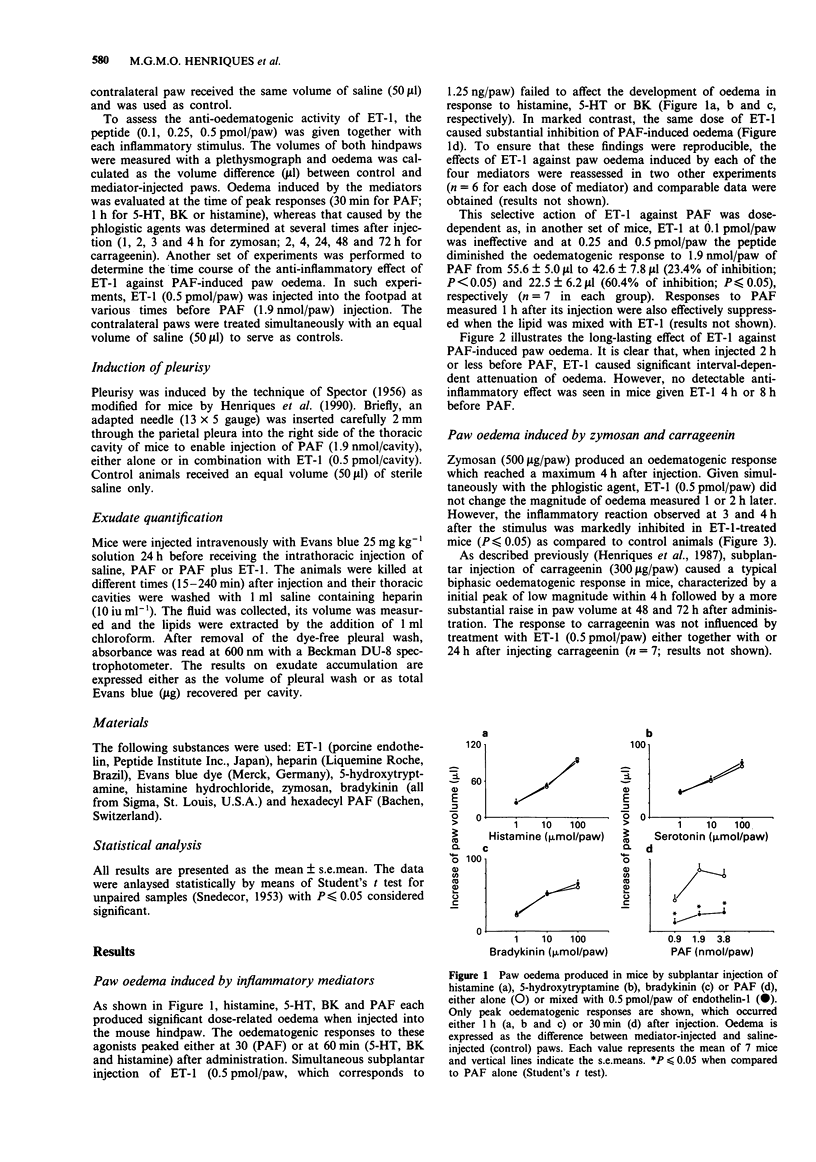
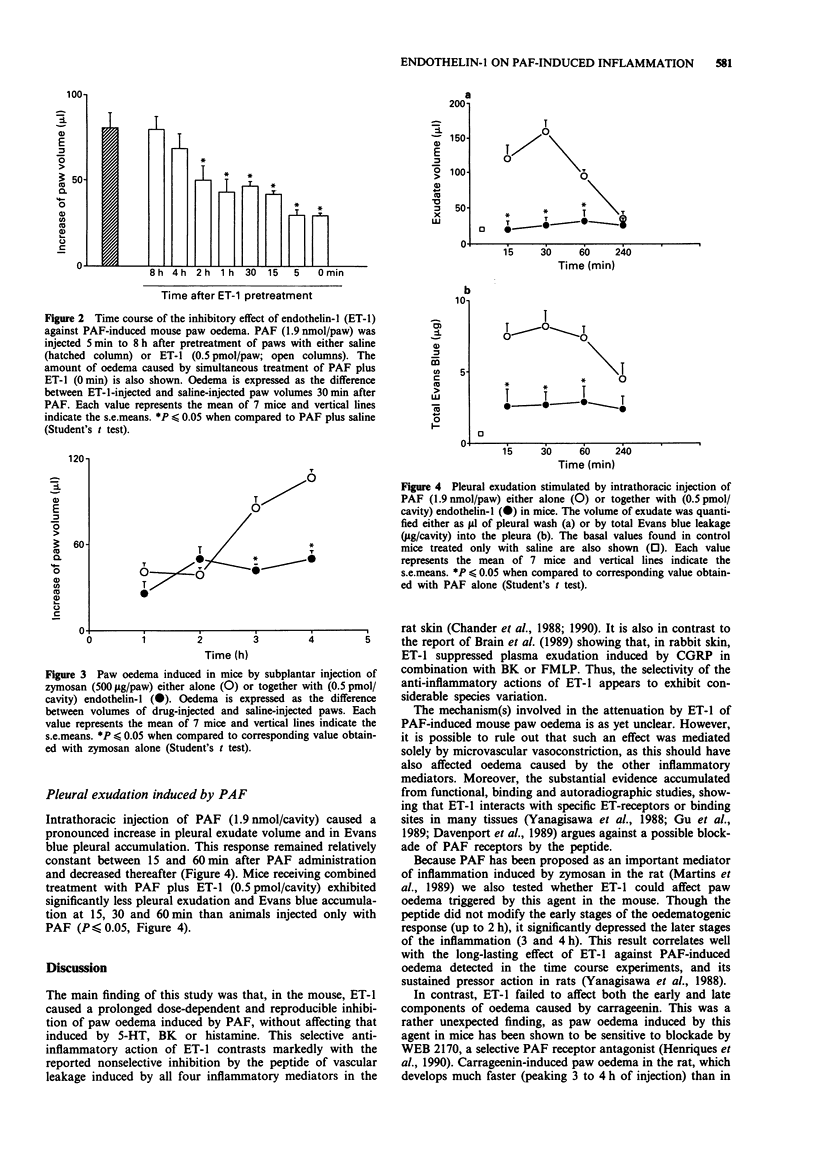
1. The current study analyses the effects of endothelin-1 (ET-1) on paw oedema and pleurisy induced by platelet activating factor (PAF) and other inflammatory agents in the mouse. 2. Combined subplantar injection of ET-1 (0.5 pmol/paw) did not modify oedema caused by histamine (1 to 100 mumol/paw), 5-hydroxytryptamine (1 to 100 mumol/paw) or bradykinin (1 to 100 nmol/paw) but markedly inhibited the response to PAF (0.95 to 3.8 nmol/paw). The selective action of ET-1 against PAF-induced (1.9 nmol/paw) oedema was dose-dependent, reaching a maximum at 0.5 pmol/paw and lasted up to 2 h. 3. ET-1 (0.5 pmol/paw) also inhibited paw oedema (3-4 h) caused by zymosan (500 micrograms/paw). In contrast, it did not modify either the early (1-4 h) or late (48-72 h) phases of the oedematogenic response to carrageenin (300 micrograms/paw), when given either together with or 24 h after the carrageenin. 4. Intrathoracic injection of PAF (1.9 nmol/cavity) induced pleurisy characterized by an increase in pleural exudate volume, and in accumulation of Evans Blue which was maximal at 30 min and lasted up to 4 h. When injected together with PAF, ET-1 (0.5 pmol/cavity) virtually abolished PAF-induced pleurisy. 5. It is concluded that ET-1 is a potent inhibitor of PAF-induced inflammation in the mouse. Its mechanism of anti-inflammatory action in this species, in contrast to what has been found in other species, does not appear to derive from its potent vasoconstrictor properties as ET-1, at the doses used, failed to affect oedematogenic responses to other inflammatory mediators.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstead W. M., Mirro R., Leffler C. W., Busija D. W. Influence of endothelin on piglet cerebral microcirculation. Am J Physiol. 1989 Aug;257(2 Pt 2):H707–H710. doi: 10.1152/ajpheart.1989.257.2.H707. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Crossman D. C., Buckley T. L., Williams T. J. Endothelin-1: demonstration of potent effects on the microcirculation of humans and other species. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S147–S150. [PubMed] [Google Scholar]
- Brain S. D., Tippins J. R., Williams T. J. Endothelin induces potent microvascular constriction. Br J Pharmacol. 1988 Dec;95(4):1005–1007. doi: 10.1111/j.1476-5381.1988.tb11731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander C. L., Howat D. W., Moore A. R., Colville-Nash P. R., Desa F. M., Braquet P., Willoughby D. A. Comparison of endothelin-1 and -3 on models of inflammation. Agents Actions. 1990 Jan;29(1-2):27–29. doi: 10.1007/BF01964710. [DOI] [PubMed] [Google Scholar]
- Chander C. L., Moore A. R., Desa F. M., Howat D., Willoughby D. A. The local modulation of vascular permeability by endothelial cell derived products. J Pharm Pharmacol. 1988 Oct;40(10):745–746. doi: 10.1111/j.2042-7158.1988.tb07011.x. [DOI] [PubMed] [Google Scholar]
- Costello A. H., Hargreaves K. M. Suppression of carrageenan-induced hyperalgesia, hyperthermia and edema by a bradykinin antagonist. Eur J Pharmacol. 1989 Nov 21;171(2-3):259–263. doi: 10.1016/0014-2999(89)90118-0. [DOI] [PubMed] [Google Scholar]
- Crunkhorn P., Meacock S. C. Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol. 1971 Jul;42(3):392–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport A. P., Nunez D. J., Hall J. A., Kaumann A. J., Brown M. J. Autoradiographical localization of binding sites for porcine [125I]endothelin-1 in humans, pigs, and rats: functional relevance in humans. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S166–S170. doi: 10.1097/00005344-198900135-00045. [DOI] [PubMed] [Google Scholar]
- Fortes Z. B., Scivoletto R., Garcia-Leme J. Endothelin-1 induces potent constriction of lymphatic vessels in situ. Eur J Pharmacol. 1989 Oct 24;170(1-2):69–73. doi: 10.1016/0014-2999(89)90135-0. [DOI] [PubMed] [Google Scholar]
- Fortes Z. B., de Nucci G., Garcia-Leme J. Effect of endothelin-1 on arterioles and venules in vivo. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S200–S201. doi: 10.1097/00005344-198900135-00056. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gu X. H., Liu J. J., Dillon J. S., Nayler W. G. The failure of endothelin to displace bound, radioactively-labelled, calcium antagonists (PN 200/110, D888 and diltiazem). Br J Pharmacol. 1989 Feb;96(2):262–264. doi: 10.1111/j.1476-5381.1989.tb11811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques M. G., Silva P. M., Martins M. A., Flores C. A., Cunha F. Q., Assreuy-Filho J., Cordeiro R. S. Mouse paw edema. A new model for inflammation? Braz J Med Biol Res. 1987;20(2):243–249. [PubMed] [Google Scholar]
- Henriques M. G., Weg V. B., Martins M. A., Silva P. M., Fernandes P. D., Cordeiro R. S., Vargaftig B. B. Differential inhibition by two hetrazepine PAF antagonists of acute inflammation in the mouse. Br J Pharmacol. 1990 Jan;99(1):164–168. doi: 10.1111/j.1476-5381.1990.tb14671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N., Tsujioka K., Tomoi M., Saida K., Mitsui Y. Differential activities of two distinct endothelin family peptides on ileum and coronary artery. FEBS Lett. 1989 Apr 24;247(2):337–340. doi: 10.1016/0014-5793(89)81365-1. [DOI] [PubMed] [Google Scholar]
- Kloog Y., Ambar I., Sokolovsky M., Kochva E., Wollberg Z., Bdolah A. Sarafotoxin, a novel vasoconstrictor peptide: phosphoinositide hydrolysis in rat heart and brain. Science. 1988 Oct 14;242(4876):268–270. doi: 10.1126/science.2845579. [DOI] [PubMed] [Google Scholar]
- Martins M. A., Silva P. M., Faria Neto H. C., Bozza P. T., Dias P. M., Lima M. C., Cordeiro R. S., Vargaftig B. B. Pharmacological modulation of Paf-induced rat pleurisy and its role in inflammation by zymosan. Br J Pharmacol. 1989 Feb;96(2):363–371. doi: 10.1111/j.1476-5381.1989.tb11826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Ohlén A., Raud J., Hedqvist P., Wiklund N. P. Microvascular effects of endothelin in the rabbit tenuissimus muscle and hamster cheek pouch. Microvasc Res. 1989 Jan;37(1):115–118. doi: 10.1016/0026-2862(89)90076-9. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Warner T. D. Simultaneous perfusion of rat isolated superior mesenteric arterial and venous beds: comparison of their vasoconstrictor and vasodilator responses to agonists. Br J Pharmacol. 1990 Feb;99(2):427–433. doi: 10.1111/j.1476-5381.1990.tb14720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]


