Abstract
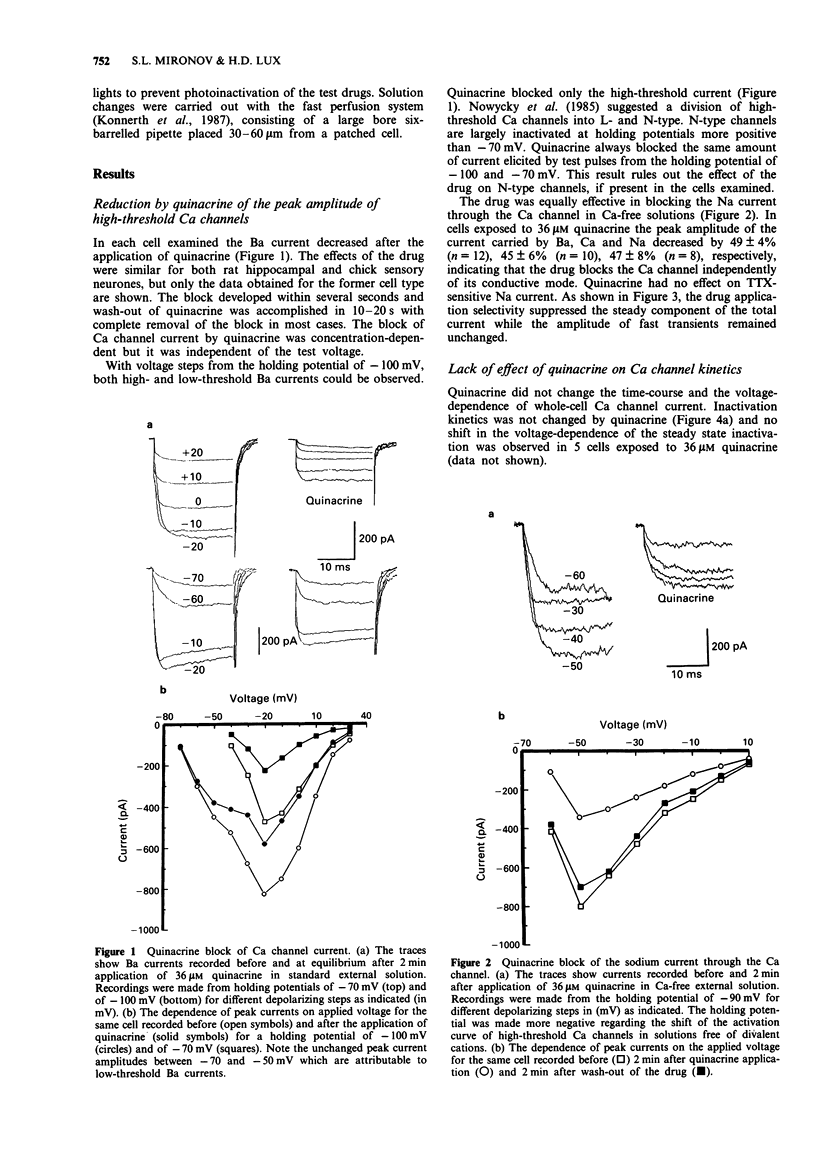
1. The whole-cell patch-clamp technique has been used to examine Ca channel currents carried by Ba (IBa) in rat hippocampal neurones. 2. Quinacrine selectivity decreased the high-threshold current activated by membrane depolarization from a holding potential of -70 mV. Neither the low-threshold Ca channel current nor the fast tetrodotoxin (TTX)-sensitive sodium current were affected by quinacrine. 3. Bath application of quinacrine caused a dose-dependent reduction of the peak amplitude of IBa. This effect was fast, voltage-independent, reversible and had a Kd of 30 +/- 5 microM. 4. The quinacrine-induced block did not change the time-course and the voltage dependence of IBa activation and deactivation. The inhibition revealed no use-dependence, ruling out an open channel block by quinacrine. 5. p-Bromophenacyl bromide had no effect on IBa suggesting the lack of involvement of phospholipase A2 in the action of quinacrine. In addition, the quinacrine-induced block was not related to the calmodulin pathway and internal quinacrine did not affect the peak amplitude of IBa. 6. The effect of quinacrine on the amplitude of IBa was dependent of the external pH, and suggested that only the single-protonated form of the drug can bind to the channel receptor with a Kd of 3 microM. Quinacrine and other substituted acridines can thus be useful for pharmacological and structure-activity studies of Ca channels.
Full text
PDF
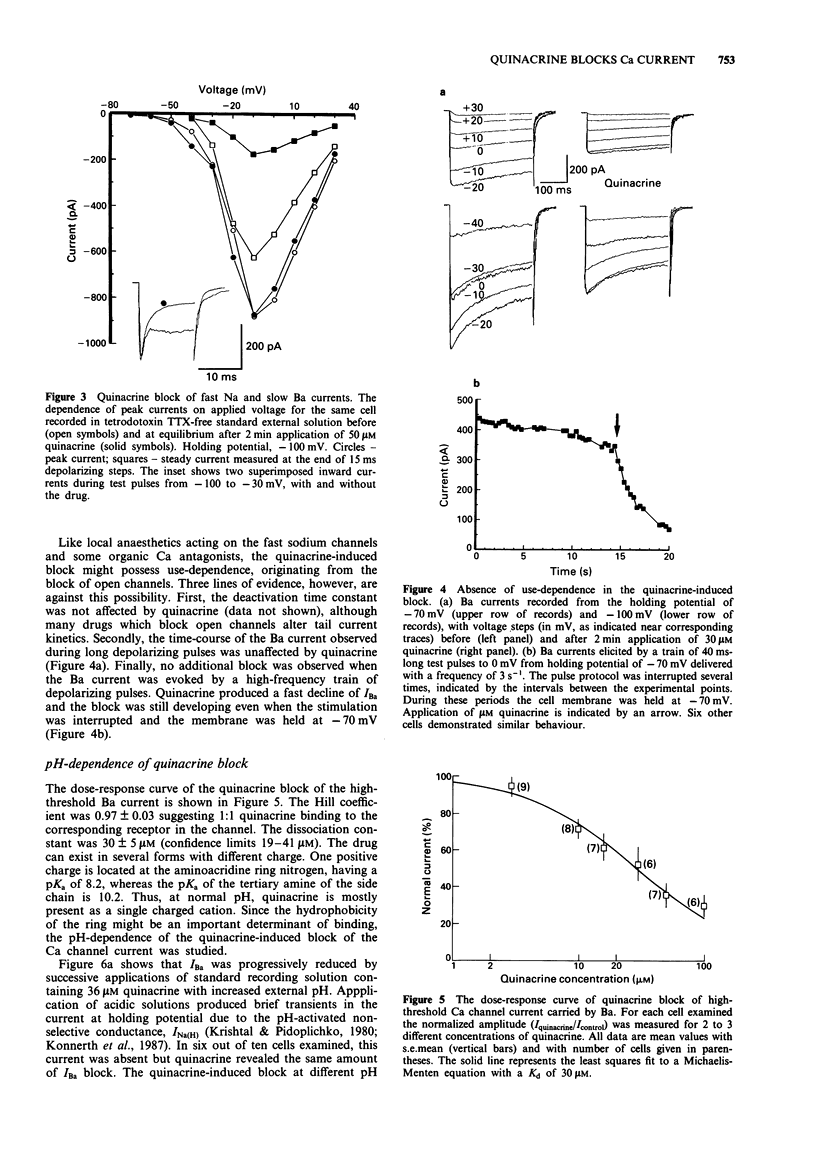
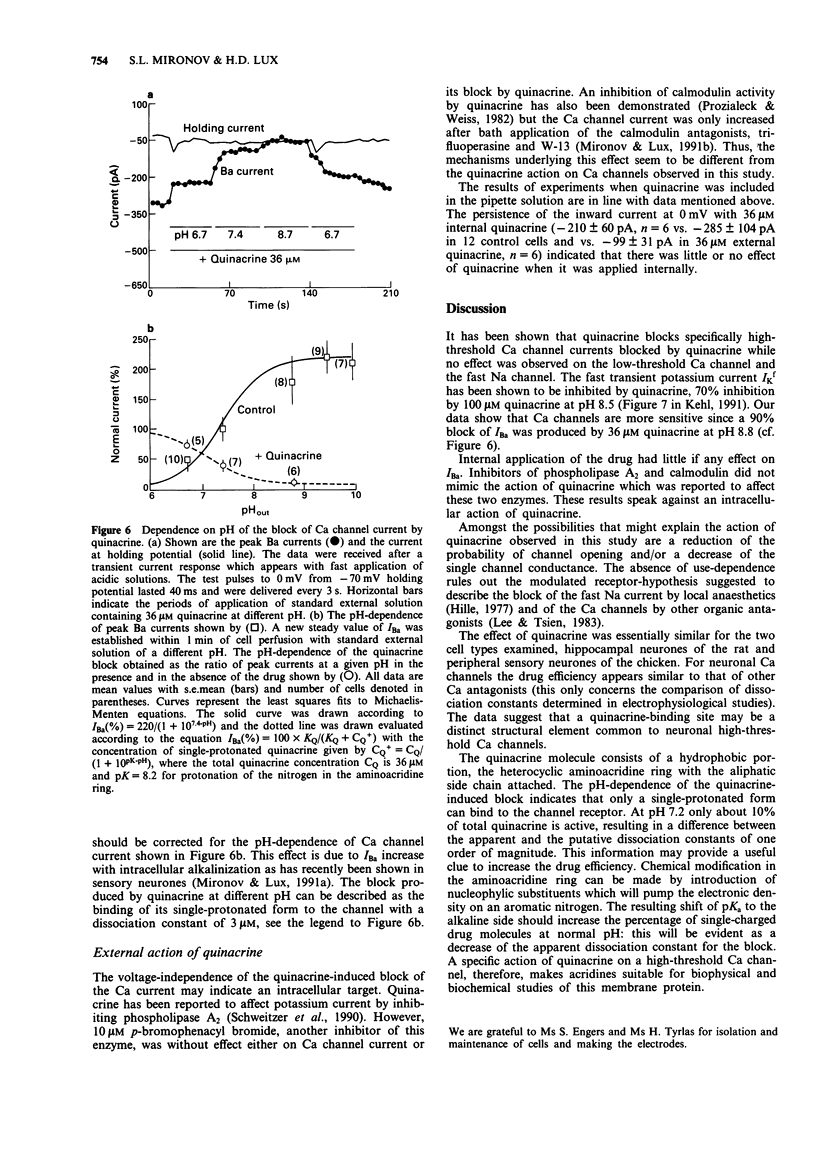

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darzynkiewicz Z., Traganos F., Kapuscinski J., Staiano-Coico L., Melamed M. R. Accessibility of DNA in situ to various fluorochromes: relationship to chromatin changes during erythroid differentiation of Friend leukemia cells. Cytometry. 1984 Jul;5(4):355–363. doi: 10.1002/cyto.990050411. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl S. J. Quinidine-induced inhibition of the fast transient outward K+ current in rat melanotrophs. Br J Pharmacol. 1991 Jul;103(3):1807–1813. doi: 10.1111/j.1476-5381.1991.tb09867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Mironov S. L., Lux H. D. Cytoplasmic alkalinization increases high-threshold calcium current in chick dorsal root ganglion neurones. Pflugers Arch. 1991 Sep;419(2):138–143. doi: 10.1007/BF00372999. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Prozialeck W. C., Weiss B. Inhibition of calmodulin by phenothiazines and related drugs: structure-activity relationships. J Pharmacol Exp Ther. 1982 Sep;222(3):509–516. [PubMed] [Google Scholar]
- Schweitzer P., Madamba S., Siggins G. R. Arachidonic acid metabolites as mediators of somatostatin-induced increase of neuronal M-current. Nature. 1990 Aug 2;346(6283):464–467. doi: 10.1038/346464a0. [DOI] [PubMed] [Google Scholar]
- Yaari Y., Hamon B., Lux H. D. Development of two types of calcium channels in cultured mammalian hippocampal neurons. Science. 1987 Feb 6;235(4789):680–682. doi: 10.1126/science.2433765. [DOI] [PubMed] [Google Scholar]


