Abstract
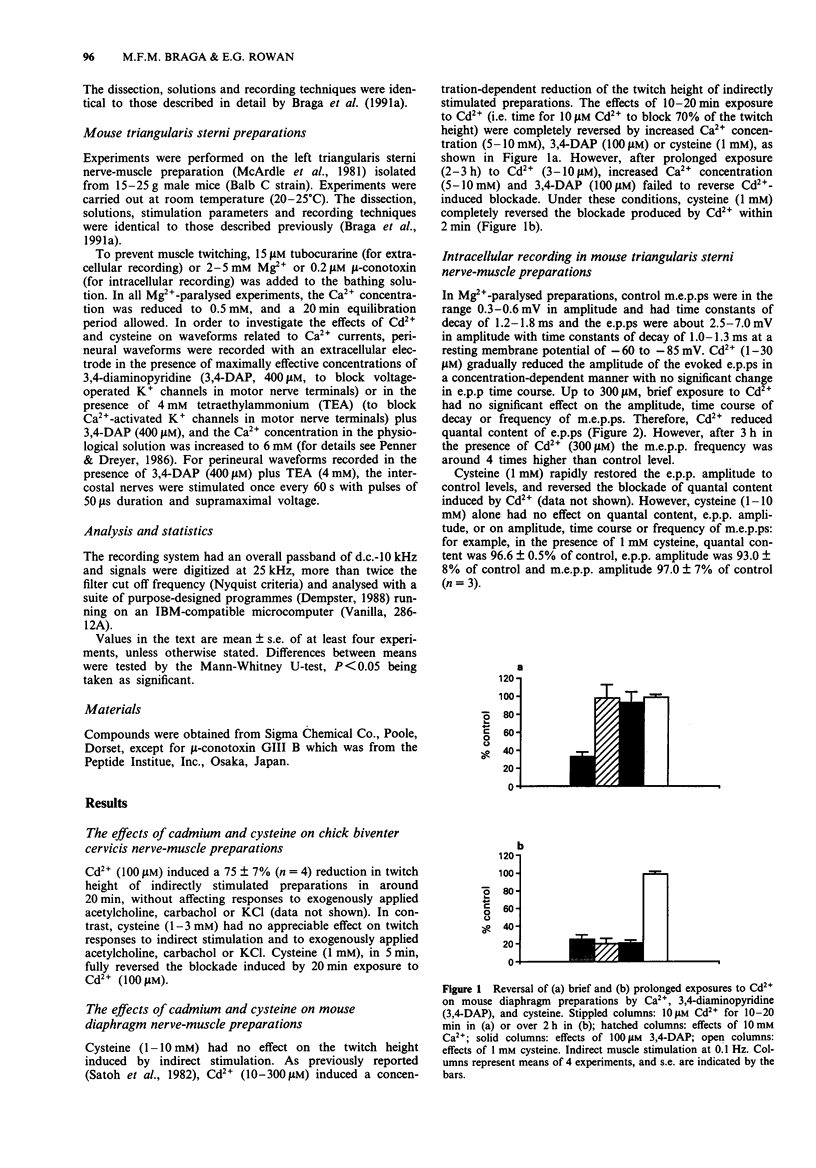
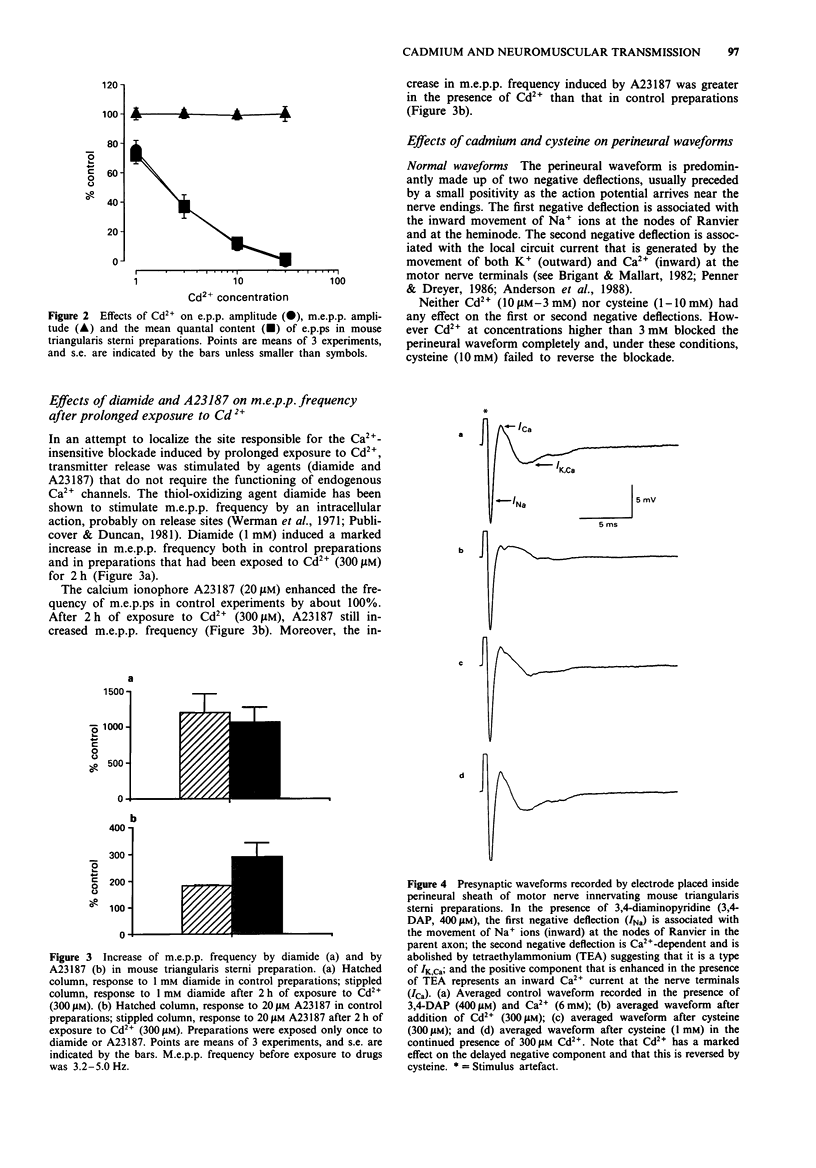
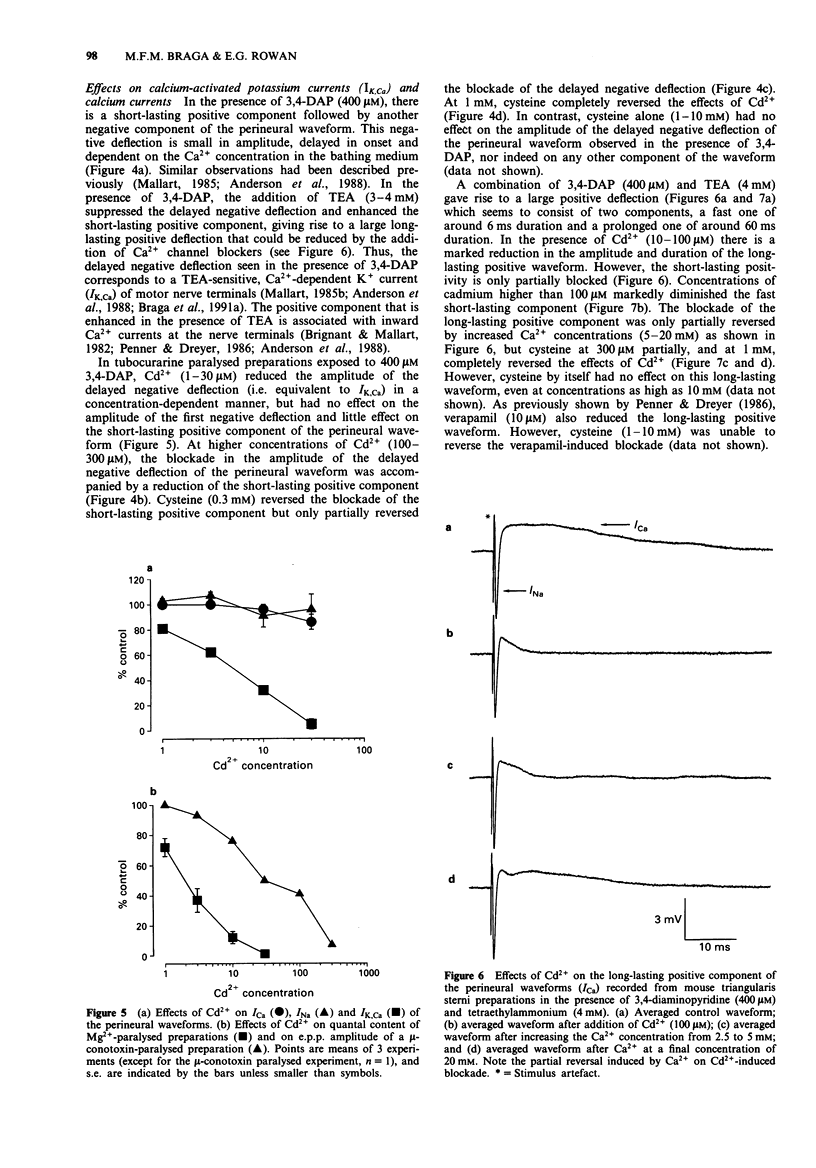
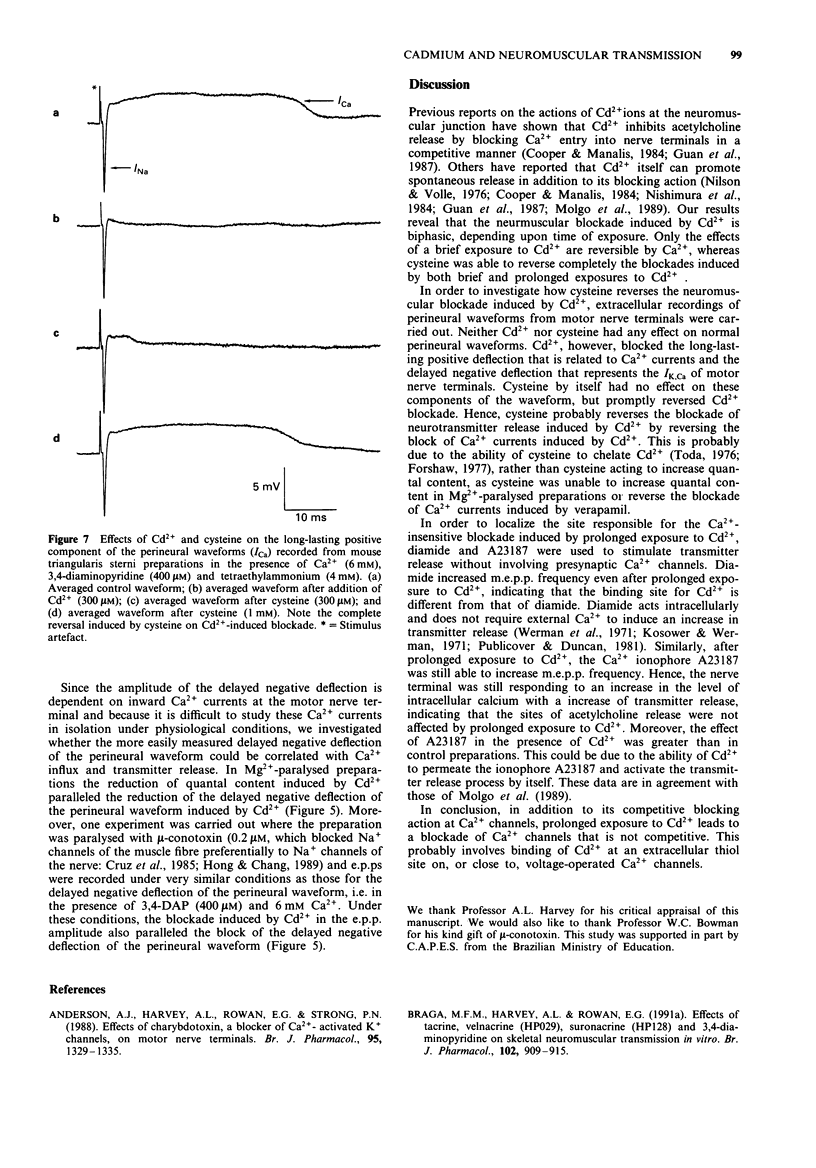
1. Neuromuscular transmission in isolated nerve-muscle preparations was blocked by exposure to Cd2+ for less than 30 min or more than 2 h. The abilities of cysteine, Ca2+ or 3,4-diaminopyridine (3,4-DAP) to reverse the blockade induced by Cd2+ were studied. 2. On the mouse hemidiaphragm preparation, exposure to Cd2+ (10 microM) for 10 to 20 min induced a blockade which was easily reversed by increasing the extracellular Ca2+ concentration (5-10 mM) or by 3,4-DAP (100 microM). Exposure to Cd2+ (3-10 microM) for over 2 h led to a blockade which was not reversed by Ca2+ (5-15 mM) or 3,4-DAP (100 microM). Cysteine (1 mM) was able to reverse completely the blockade induced by both brief and prolonged exposures to Cd2+. 3. In chick biventer cervicis preparations, Cd2+ (100 microM) decreased the twitch height of indirectly stimulated preparations without affecting responses to exogenously applied acetylcholine, carbachol or KCl. Cysteine (1-3 mM) had no appreciable effect on twitch responses to indirect stimulation or to exogenously applied agonists but fully reversed the blockade induced by Cd2+ (100 microM). 4. In mouse triangularis sterni preparations, Cd2+ (1-30 microM) depressed the evoked quantal release of acetylcholine. Concentrations of Cd2+ which completely blocked endplate potentials (e.p.ps) were without significant effect on miniature endplate potential (m.e.p.p.) amplitude and frequency or time constant of decay. Cysteine (1-10 mM) alone had no effect on e.p.ps or m.e.p.ps, but completely reversed the blockade induced by Cd2+.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Harvey A. L., Rowan E. G., Strong P. N. Effects of charybdotoxin, a blocker of Ca2+-activated K+ channels, on motor nerve terminals. Br J Pharmacol. 1988 Dec;95(4):1329–1335. doi: 10.1111/j.1476-5381.1988.tb11772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga M. F., Harvey A. L., Rowan E. G. Effects of tacrine, velnacrine (HP029), suronacrine (HP128), and 3,4-diaminopyridine on skeletal neuromuscular transmission in vitro. Br J Pharmacol. 1991 Apr;102(4):909–915. doi: 10.1111/j.1476-5381.1991.tb12275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen P. L., Kosower E. M., Werman R. Diamide acts intracellularly to enhance transmitter release: the differential permeation of diamide, DIP, DIP+1 and DIP+2 across the nerve terminal membrane. Brain Res. 1976 Nov 26;117(2):277–285. doi: 10.1016/0006-8993(76)90735-6. [DOI] [PubMed] [Google Scholar]
- Carlen P. L., Kosower E. M., Werman R. The thiol-oxidizing agent diamide increases transmitter release by decreasing calcium requirements for neuromuscular transmission in the frog. Brain Res. 1976 Nov 26;117(2):257–276. doi: 10.1016/0006-8993(76)90734-4. [DOI] [PubMed] [Google Scholar]
- Cooper G. P., Manalis R. S. Cadmium: effects on transmitter release at the frog neuromuscular junction. Eur J Pharmacol. 1984 Apr 6;99(4):251–256. doi: 10.1016/0014-2999(84)90131-6. [DOI] [PubMed] [Google Scholar]
- Cruz L. J., Gray W. R., Olivera B. M., Zeikus R. D., Kerr L., Yoshikami D., Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985 Aug 5;260(16):9280–9288. [PubMed] [Google Scholar]
- Forshaw P. J. The inhibitory effect of cadmium on neuromuscular transmission in the rat. Eur J Pharmacol. 1977 Apr 21;42(4):371–377. doi: 10.1016/0014-2999(77)90171-6. [DOI] [PubMed] [Google Scholar]
- GINSBORG B. L., WARRINER J. The isolated chick biventer cervicis nerve-muscle preparation. Br J Pharmacol Chemother. 1960 Sep;15:410–411. doi: 10.1111/j.1476-5381.1960.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y. Y., Quastel D. M., Saint D. A. Multiple actions of cadmium on transmitter release at the mouse neuromuscular junction. Can J Physiol Pharmacol. 1987 Oct;65(10):2131–2136. doi: 10.1139/y87-334. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. Br J Pharmacol. 1989 Jul;97(3):934–940. doi: 10.1111/j.1476-5381.1989.tb12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower E. M., Werman R. New step in transmitter release at the myoneural junction. Nat New Biol. 1971 Sep 22;233(38):121–123. doi: 10.1038/newbio233121a0. [DOI] [PubMed] [Google Scholar]
- Lin-Shiau S. Y., Fu W. M. Effects of divalent cations on neuromuscular transmission in the chick. Eur J Pharmacol. 1980 Jun 27;64(4):259–269. doi: 10.1016/0014-2999(80)90233-2. [DOI] [PubMed] [Google Scholar]
- Mallart A. Electric current flow inside perineurial sheaths of mouse motor nerves. J Physiol. 1985 Nov;368:565–575. doi: 10.1113/jphysiol.1985.sp015876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J., Angaut-Petit D., Mallart A., Bournaud R., Faille L., Brigant J. L. Advantages of the triangularis sterni muscle of the mouse for investigations of synaptic phenomena. J Neurosci Methods. 1981 Aug;4(2):109–115. doi: 10.1016/0165-0270(81)90044-3. [DOI] [PubMed] [Google Scholar]
- Molgó J., Pécot-Dechavassine M., Thesleff S. Effects of cadmium on quantal transmitter release and ultrastructure of frog motor nerve endings. J Neural Transm. 1989;77(2-3):79–91. doi: 10.1007/BF01248924. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Tsutsui I., Yagasaki O., Yanagiya I. Transmitter release at the mouse neuromuscular junction stimulated by cadmium ions. Arch Int Pharmacodyn Ther. 1984 Sep;271(1):106–121. [PubMed] [Google Scholar]
- Penner R., Dreyer F. Two different presynaptic calcium currents in mouse motor nerve terminals. Pflugers Arch. 1986 Feb;406(2):190–197. doi: 10.1007/BF00586682. [DOI] [PubMed] [Google Scholar]
- Publicover S. J., Duncan C. J. Diamide, temperature and spontaneous transmitter release at the neuromuscular junction: stimulation of exocytosis by a direct effect on membrane fusion? Eur J Pharmacol. 1981 Mar 12;70(2):203–211. doi: 10.1016/0014-2999(81)90215-6. [DOI] [PubMed] [Google Scholar]
- Satoh E., Asai F., Itoh K., Nishimura M., Urakawa N. Mechanism of cadmium-induced blockade of neuromuscular transmission. Eur J Pharmacol. 1982 Feb 5;77(4):251–257. doi: 10.1016/0014-2999(82)90126-1. [DOI] [PubMed] [Google Scholar]
- Toda N. Neuromuscular blocking action of cadmium and manganese in isolated frog striated muscles. Eur J Pharmacol. 1976 Nov;40(1):67–75. doi: 10.1016/0014-2999(76)90355-1. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Werman R., Carlen P. L., Kushnir M., Kosower E. M. Effect of the thiol-oxidizing agent, diamide, on acetylcholine release at the frog endplate. Nat New Biol. 1971 Sep 22;233(38):120–121. doi: 10.1038/newbio233120a0. [DOI] [PubMed] [Google Scholar]


