Abstract
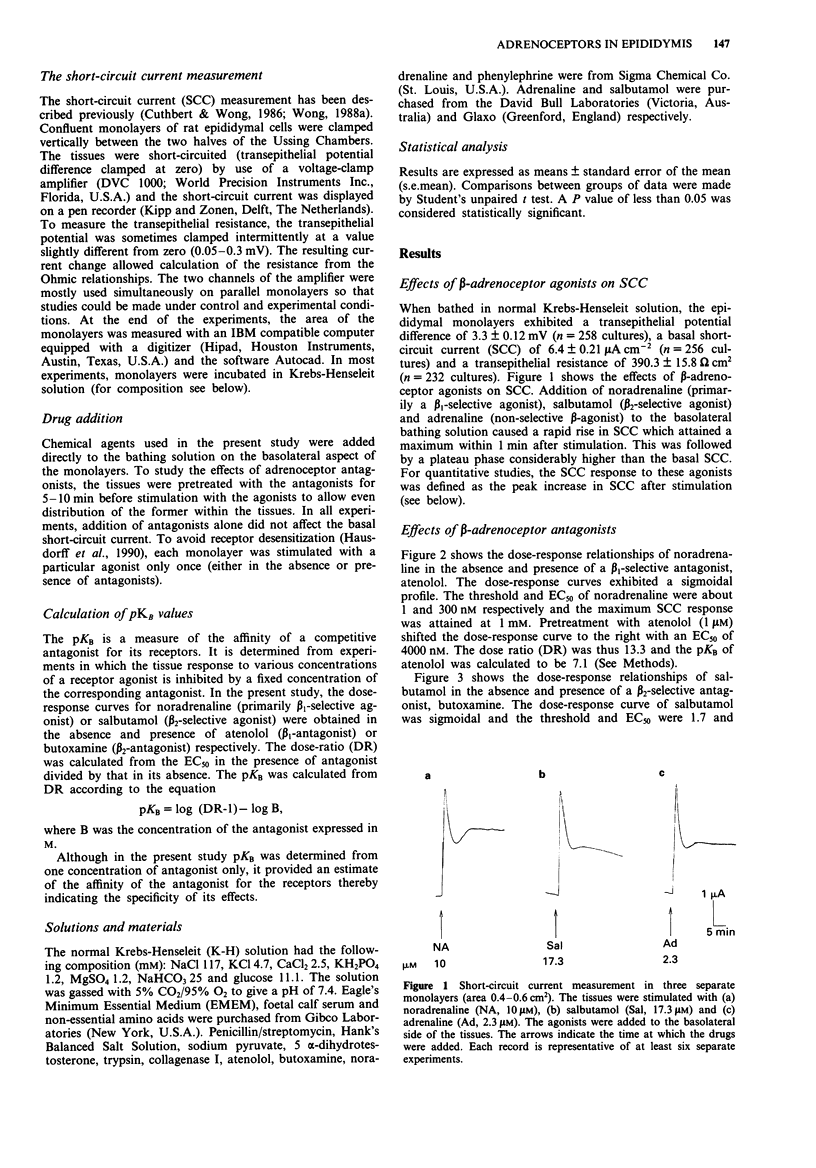
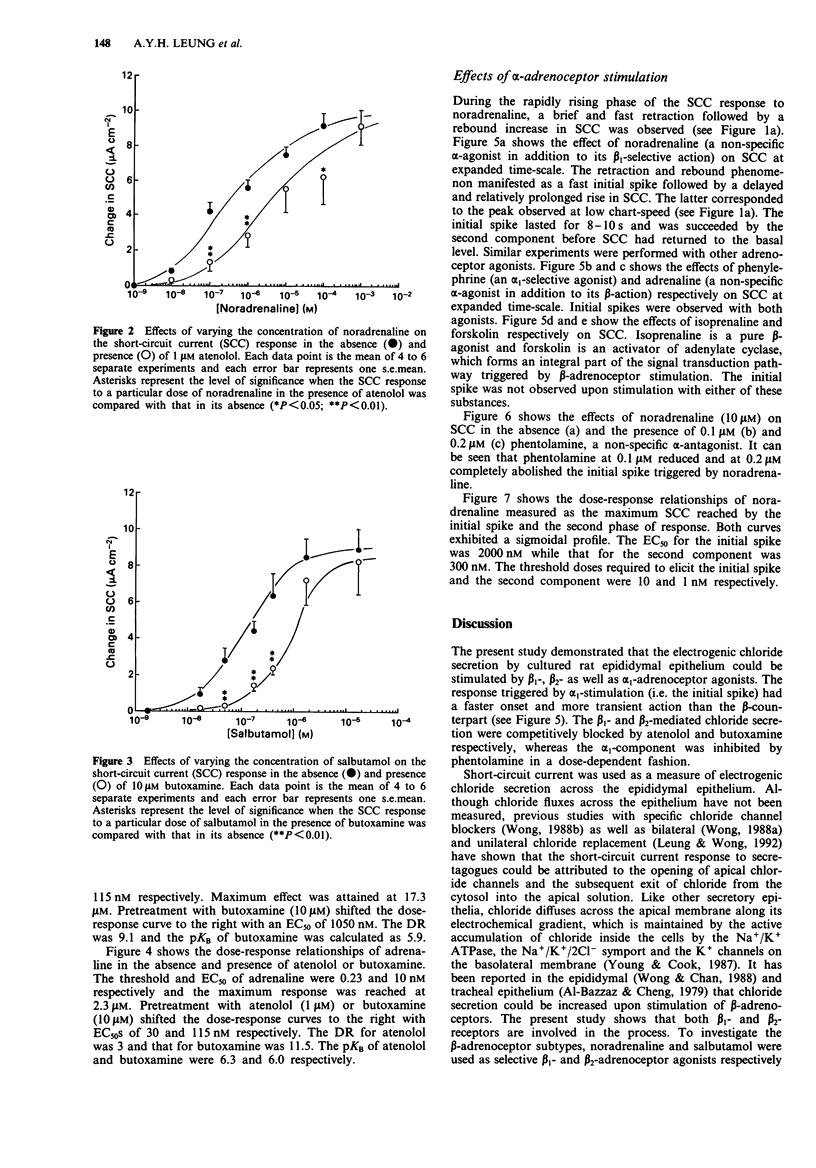
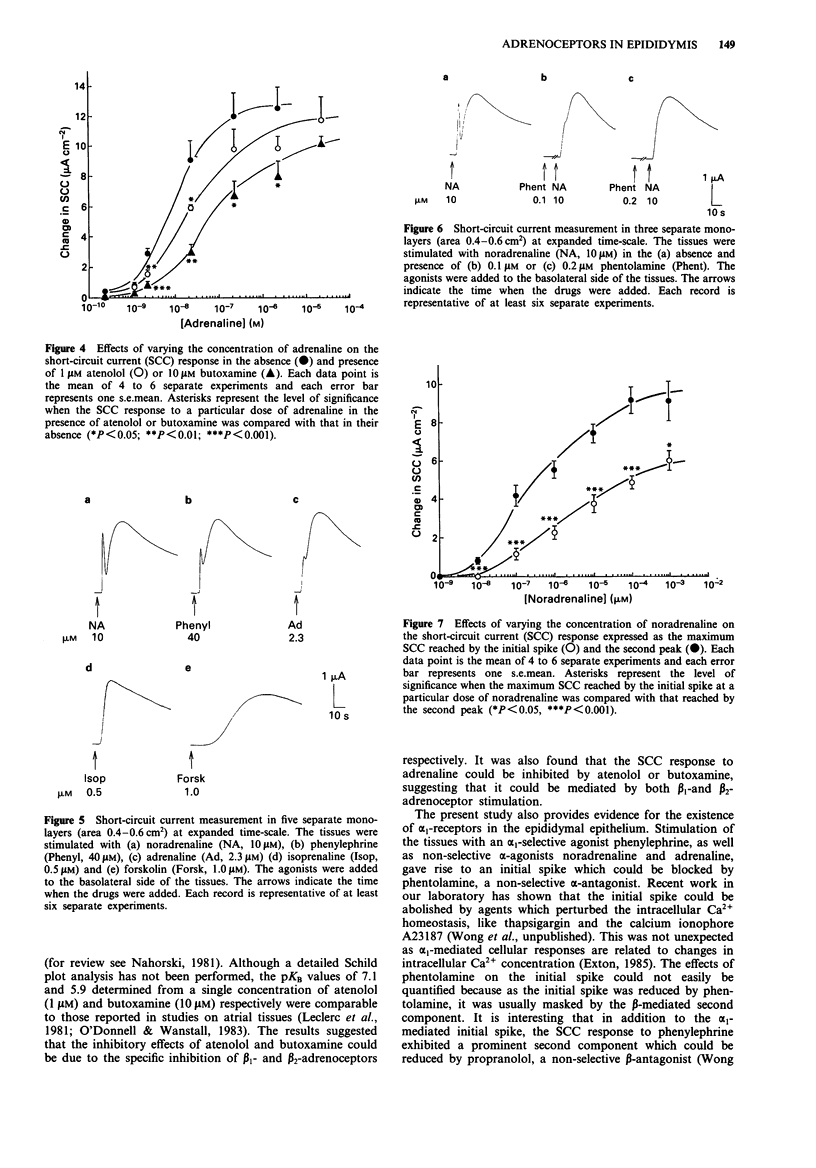
1. Short-circuit current (SCC) technique was used to study the adrenoceptors involved in the electrogenic chloride secretion by cultured cauda epididymal epithelium of rats. Stimulation of the epithelium with noradrenaline (primarily beta 1-adrenoceptor selective agonist), salbutamol (beta 2-adrenoceptor selective agonist) and adrenaline (non-selective beta-adrenoceptor agonist) led to a rise in SCC. At a low chart-speed (2 mm min-1), the response profile to these agonists consisted of a peak followed by a sustained response considerably higher than the basal SCC. 2. The EC50s (doses of agonist producing 50% maximum response) of noradrenaline, salbutamol and adrenaline were 300, 115 and 10 nM respectively. Pretreating the tissues with 1 microM atenolol (beta 1-selective antagonist) and 10 microM butoxamine (beta 2-selective antagonist) shifted the dose-response curves of noradrenaline (shifted EC50 = 4000 nM) and salbutamol (shifted EC50 = 1050 nM) to the right. Atenolol (1 microM) and butoxamine (10 microM) shifted the dose-response curve of adrenaline to the right with new EC50s of 30 nM and 115 nM, respectively. 3. The rapidly rising phase of the SCC response to noradrenaline and adrenaline observed at low chart-speed consisted of a brief and transient retraction followed by a rebound increase in SCC. At a high chart-speed (1 mm s-1), the retraction and rebound phenomenon manifested as a fast initial spike which could be blocked by phentolamine (non-specific alpha-adrenoceptor antagonist) in a dose-dependent fashion.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bazzaz F. J., Cheng E. Effect of catecholamines on ion transport in dog tracheal epithelium. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):397–403. doi: 10.1152/jappl.1979.47.2.397. [DOI] [PubMed] [Google Scholar]
- Bainbridge T., Feldman R. D., Welsh M. J. Adrenergic stimulation of inositol phosphate accumulation in tracheal epithelium. J Appl Physiol (1985) 1989 Jan;66(1):504–508. doi: 10.1152/jappl.1989.66.1.504. [DOI] [PubMed] [Google Scholar]
- Billups K. L., Tillman S. L., Chang T. S. Reduction of epididymal sperm motility after ablation of the inferior mesenteric plexus in the rat. Fertil Steril. 1990 Jun;53(6):1076–1082. [PubMed] [Google Scholar]
- Billups K. L., Tillman S., Chang T. S. Ablation of the inferior mesenteric plexus in the rat: alteration of sperm storage in the epididymis and vas deferens. J Urol. 1990 Mar;143(3):625–629. doi: 10.1016/s0022-5347(17)40043-7. [DOI] [PubMed] [Google Scholar]
- Breuer W. V., Mack E., Rothstein A. Activation of K+ and Cl- channels by Ca2+ and cyclic AMP in dissociated kidney epithelial (MDCK) cells. Pflugers Arch. 1988 Apr;411(4):450–455. doi: 10.1007/BF00587726. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Wong P. Y. Electrogenic anion secretion in cultured rat epididymal epithelium. J Physiol. 1986 Sep;378:335–345. doi: 10.1113/jphysiol.1986.sp016222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. B., Silski C. L., Kercsmar C. M., Infeld M. Beta-adrenergic receptors on human tracheal epithelial cells in primary culture. Am J Physiol. 1990 Jan;258(1 Pt 1):C71–C76. doi: 10.1152/ajpcell.1990.258.1.C71. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Welsh M. J. Ca2+ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol. 1986;48:135–150. doi: 10.1146/annurev.ph.48.030186.001031. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G., Lambie D. G., Johnson R. H. Beta-adrenoceptor responsiveness and plasma catecholamines as determinants of cardiovascular reactivity to mental stress. Clin Sci (Lond) 1985 Oct;69(4):483–492. doi: 10.1042/cs0690483. [DOI] [PubMed] [Google Scholar]
- El-Badawi A., Schenk E. A. The distribution of cholinergic and adrenergic nerves in the mammalian epididymis: a comparative histochemical study. Am J Anat. 1967 Jul;121(1):1–14. doi: 10.1002/aja.1001210102. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Rechkemmer G. R., Schoumacher R. A., Frizzell R. A. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988 Apr;254(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Jenkins A. D., Lechene C. P., Howards S. S. Concentrations of seven elements in the intraluminal fluids of the rat seminiferous tubules, rate testis, and epididymis. Biol Reprod. 1980 Dec;23(5):981–987. doi: 10.1095/biolreprod23.5.981. [DOI] [PubMed] [Google Scholar]
- Levitzki A. From epinephrine to cyclic AMP. Science. 1988 Aug 12;241(4867):800–806. doi: 10.1126/science.2841758. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Basolateral K+ channels in airway epithelia. II. Role in Cl- secretion and evidence for two types of K+ channel. Am J Physiol. 1990 Jun;258(6 Pt 1):L343–L348. doi: 10.1152/ajplung.1990.258.6.L343. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Relaxation of cat trachea by beta-adrenoceptor agonists can be mediated by both beta1- and beta2-adrenoceptors and potentiated by inhibitors of extraneuronal uptake. Br J Pharmacol. 1983 Feb;78(2):417–424. doi: 10.1111/j.1476-5381.1983.tb09406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Paulmichl M., Wöll E., Paulmichl R., Lang F. Cellular mechanisms of adrenaline-induced hyperpolarization in renal epitheloid MDCK cells. Biochem J. 1991 Feb 15;274(Pt 1):243–248. doi: 10.1042/bj2740243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard C. E., Harris A., Coleman L., Argent B. E. Chloride channels on epithelial cells cultured from human fetal epididymis. J Membr Biol. 1991 Dec;124(3):275–284. doi: 10.1007/BF01994360. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Stimulus-secretion coupling: general models and specific aspects in epithelial cells. Methods Enzymol. 1990;191:661–676. doi: 10.1016/0076-6879(90)91040-d. [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989 Oct 15;264(29):17131–17141. [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Alpha 1-adrenergic receptor-mediated phosphoinositide hydrolysis and prostaglandin E2 formation in Madin-Darby canine kidney cells. Possible parallel activation of phospholipase C and phospholipase A2. J Biol Chem. 1987 Mar 25;262(9):4200–4207. [PubMed] [Google Scholar]
- Smith P. L., Welsh M. J., Stoff J. S., Frizzell R. A. Chloride secretion by canine tracheal epithelium: I. Role of intracellular c AMP levels. J Membr Biol. 1982;70(3):217–226. doi: 10.1007/BF01870564. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Huang S. J. Secretory agonists stimulate a rise in intracellular cyclic AMP but not Ca2+ and inositol phosphates in cultured rat epididymal epithelium. Exp Physiol. 1990 May;75(3):321–337. doi: 10.1113/expphysiol.1990.sp003407. [DOI] [PubMed] [Google Scholar]
- Wong P. Y. Inhibition by chloride channel blockers of anion secretion in cultured epididymal epithelium and intact epididymis of rats. Br J Pharmacol. 1988 May;94(1):155–163. doi: 10.1111/j.1476-5381.1988.tb11510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. Y. Mechanism of adrenergic stimulation of anion secretion in cultured rat epididymal epithelium. Am J Physiol. 1988 Jan;254(1 Pt 2):F121–F133. doi: 10.1152/ajprenal.1988.254.1.F121. [DOI] [PubMed] [Google Scholar]


