Abstract
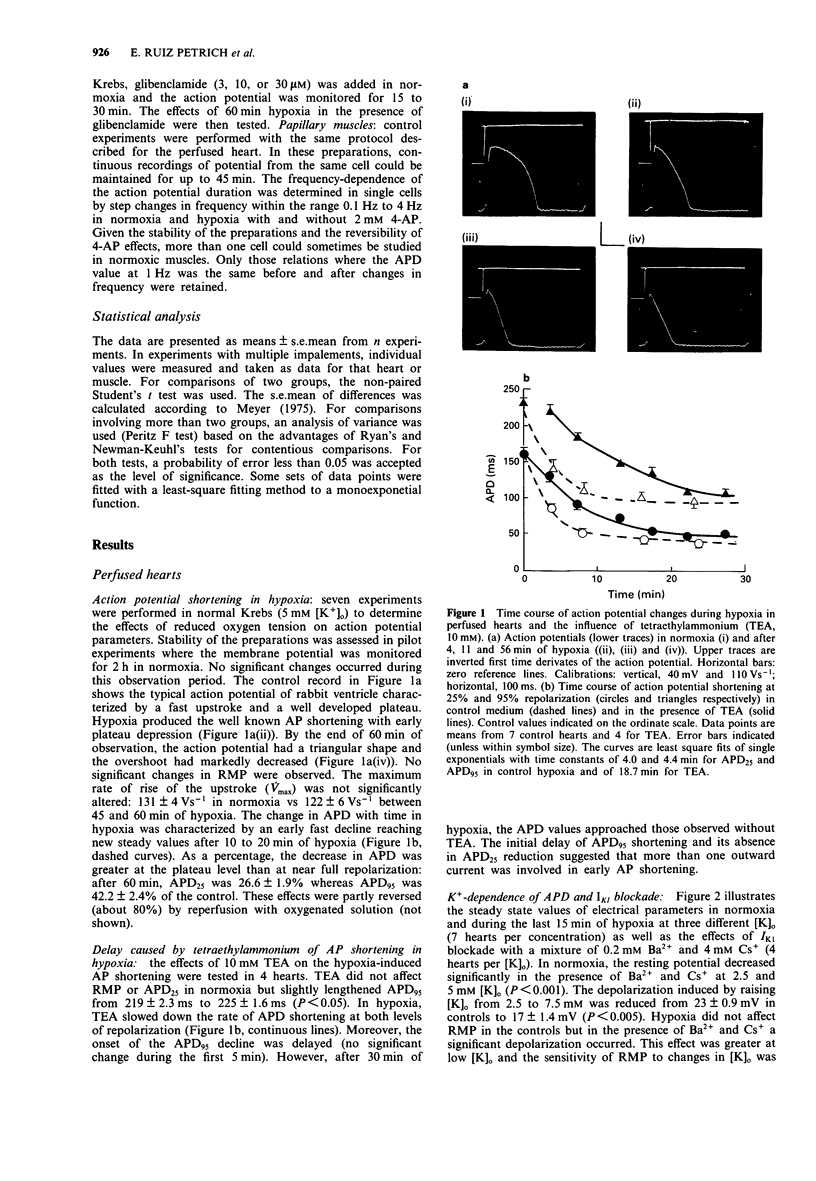
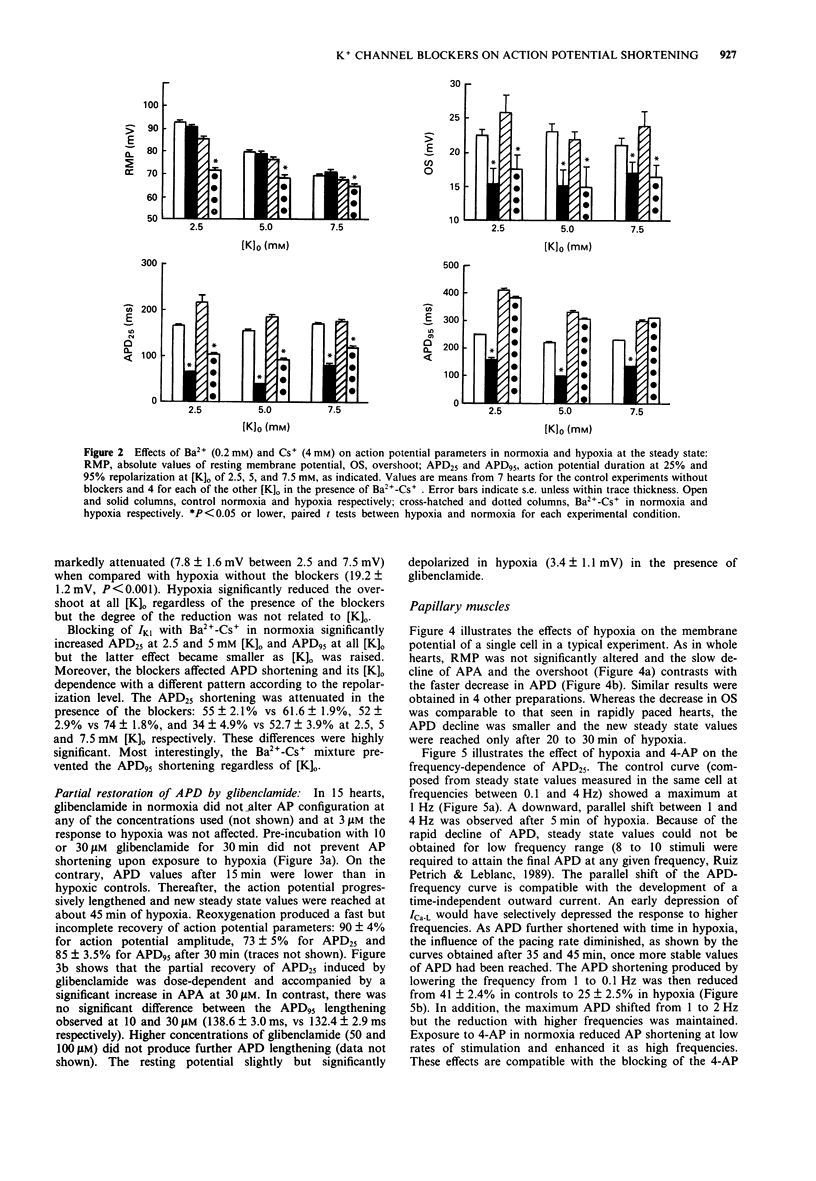
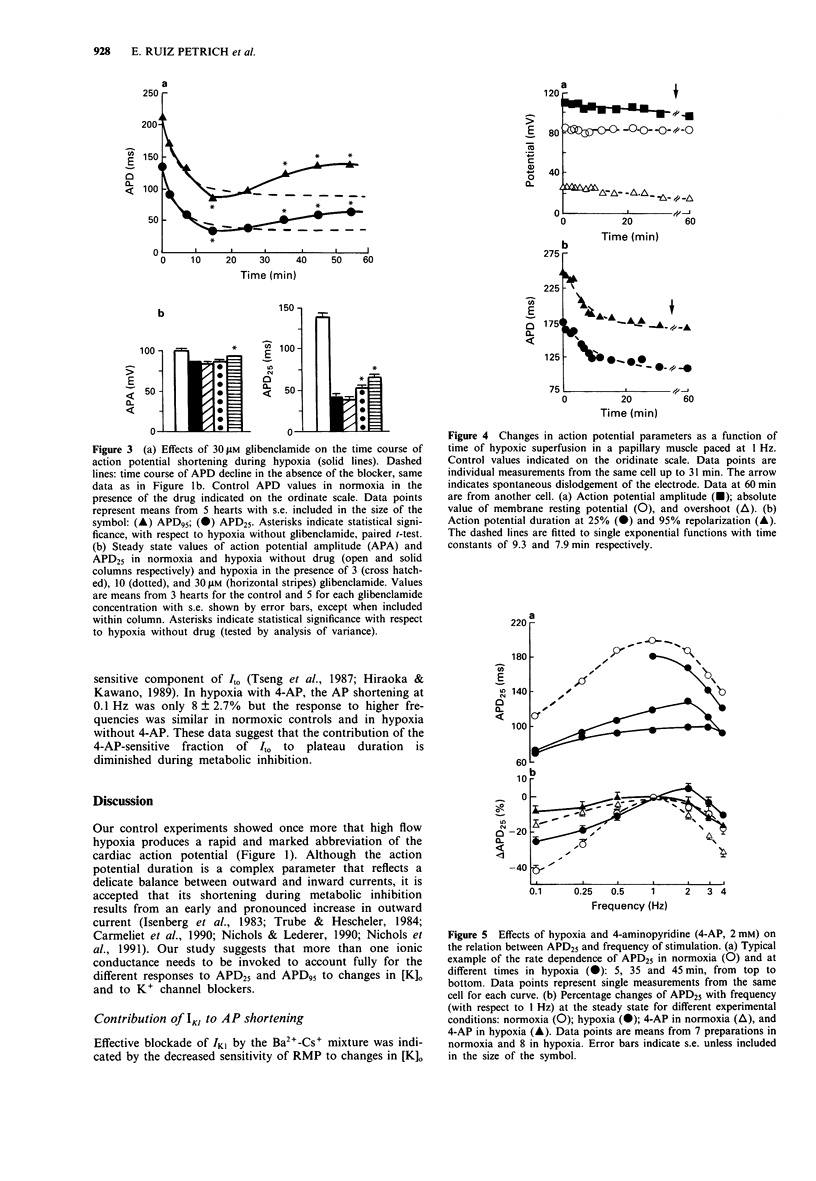
1. In order to assess the role of different ionic currents in hypoxia-induced action potential shortening, we investigated the effects of blockers of voltage-dependent and ATP-sensitive K(+)-channel on the membrane potential of hypoxic rabbit hearts and papillary muscles. The response to blocking of the inward rectifier was studied at three external K+ concentration: 2.5, 5, and 7.5 mM. 2. Hypoxia produced a progressive decline in action potential duration (APD) that levelled off after 15 to 20 min. Steady state APD values at 25% and 95% repolarization (APD25 and APD95) were 26.0 +/- 1.9% and 42.2 +/- 2.4% of controls respectively. 3. Tetraethylammonium (TEA, 10 mM) delayed but did not reduce APD shortening at the steady state. 4. Blocking of IK1 with a mixture of 0.2 mM Ba2+ and 4 mM Cs+ lengthened APD in normoxia and prevented APD95 shortening in hypoxia. The APD25 shortening was significantly attenuated at all [K]o. 5. Glibenclamide (Glib, 30 microM) did not prevent APD shortening, but produced a progressive action potential (AP) lengthening after 15 min of hypoxia. Steady levels of 48 +/- 3.5% and 62 +/- 5.0% of controls for APD25 and APD95 respectively were reached after 45 min. 6. The relation between APD25 and pacing rate was determined in normoxic and hypoxic papillary muscles and the effects of 2 mM 4-aminopyridine (4-AP) were examined. Hypoxia attenuated the APD25 shortening currently observed when the stimulation rate was lowered from 1 to 0.1 Hz without altering the plateau reduction occurring at frequencies above 2 Hz. These effects were potentiated by 4-AP.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arena J. P., Kass R. S. Activation of ATP-sensitive K channels in heart cells by pinacidil: dependence on ATP. Am J Physiol. 1989 Dec;257(6 Pt 2):H2092–H2096. doi: 10.1152/ajpheart.1989.257.6.H2092. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Cardiac transmembrane potentials and metabolism. Circ Res. 1978 May;42(5):577–587. doi: 10.1161/01.res.42.5.577. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Allen D. G. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989 Mar;64(3):583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- Findlay I., Faivre J. F. ATP-sensitive K channels in heart muscle. Spare channels. FEBS Lett. 1991 Feb 11;279(1):95–97. doi: 10.1016/0014-5793(91)80259-6. [DOI] [PubMed] [Google Scholar]
- Fosset M., De Weille J. R., Green R. D., Schmid-Antomarchi H., Lazdunski M. Antidiabetic sulfonylureas control action potential properties in heart cells via high affinity receptors that are linked to ATP-dependent K+ channels. J Biol Chem. 1988 Jun 15;263(17):7933–7936. [PubMed] [Google Scholar]
- Gasser R. N., Vaughan-Jones R. D. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J Physiol. 1990 Dec;431:713–741. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks M. N., Cobbe S. M. Effect of glibenclamide on extracellular potassium accumulation and the electrophysiological changes during myocardial ischaemia in the arterially perfused interventricular septum of rabbit. Cardiovasc Res. 1991 May;25(5):407–413. doi: 10.1093/cvr/25.5.407. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Hiraoka M. Barium-induced automatic activity in isolated ventricular myocytes from guinea-pig hearts. J Physiol. 1988 Jan;395:455–472. doi: 10.1113/jphysiol.1988.sp016929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol. 1989 Mar;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Mechanism of increased amplitude and duration of the plateau with sudden shortening of diastolic intervals in rabbit ventricular cells. Circ Res. 1987 Jan;60(1):14–26. doi: 10.1161/01.res.60.1.14. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Vereecke J., van der Heyden G., Carmeliet E. The shortening of the action potential by DNP in guinea-pig ventricular myocytes is mediated by an increase of a time-independent K conductance. Pflugers Arch. 1983 Jun 1;397(4):251–259. doi: 10.1007/BF00580257. [DOI] [PubMed] [Google Scholar]
- Jiang C., Crake T., Poole-Wilson P. A. Inhibition by barium and glibenclamide of the net loss of 86Rb+ from rabbit myocardium during hypoxia. Cardiovasc Res. 1991 May;25(5):414–420. doi: 10.1093/cvr/25.5.414. [DOI] [PubMed] [Google Scholar]
- Leblanc N., Ruiz-Ceretti E., Chartier D. Potassium loss from hypoxic myocardium: influence of external K concentration. Can J Physiol Pharmacol. 1987 May;65(5):861–866. doi: 10.1139/y87-138. [DOI] [PubMed] [Google Scholar]
- Leblanc N., Ruiz-Ceretti E. The diffusion and electrogenic components of the membrane potential of hypoxic myocardium. Can J Physiol Pharmacol. 1987 Feb;65(2):246–251. doi: 10.1139/y87-043. [DOI] [PubMed] [Google Scholar]
- Nakaya H., Takeda Y., Tohse N., Kanno M. Effects of ATP-sensitive K+ channel blockers on the action potential shortening in hypoxic and ischaemic myocardium. Br J Pharmacol. 1991 May;103(1):1019–1026. doi: 10.1111/j.1476-5381.1991.tb12294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990 Apr;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. G., Ripoll C., Lederer W. J. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Petrich E., Leblanc N. The mechanism of the rate-dependent changes of the conducted action potential in rabbit ventricle. Can J Physiol Pharmacol. 1989 Jul;67(7):780–787. doi: 10.1139/y89-124. [DOI] [PubMed] [Google Scholar]
- Ruiz-Petrich E., de Lorenzi F., Chartier D. Role of the inward rectifier IK1 in the myocardial response to hypoxia. Cardiovasc Res. 1991 Jan;25(1):17–26. doi: 10.1093/cvr/25.1.17. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Silverman H. S., Houser S. R., Josephson R. A., Capogrossi M. C., Nichols C. G., Lederer W. J., Lakatta E. G. Anoxic contractile failure in rat heart myocytes is caused by failure of intracellular calcium release due to alteration of the action potential. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6954–6958. doi: 10.1073/pnas.85.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Robinson R. B., Hoffman B. F. Passive properties and membrane currents of canine ventricular myocytes. J Gen Physiol. 1987 Nov;90(5):671–701. doi: 10.1085/jgp.90.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. N., Lamp S. T. Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science. 1987 Oct 2;238(4823):67–69. doi: 10.1126/science.2443972. [DOI] [PubMed] [Google Scholar]
- Wilde A. A., Escande D., Schumacher C. A., Thuringer D., Mestre M., Fiolet J. W., Janse M. J. Potassium accumulation in the globally ischemic mammalian heart. A role for the ATP-sensitive potassium channel. Circ Res. 1990 Oct;67(4):835–843. doi: 10.1161/01.res.67.4.835. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]


