Abstract
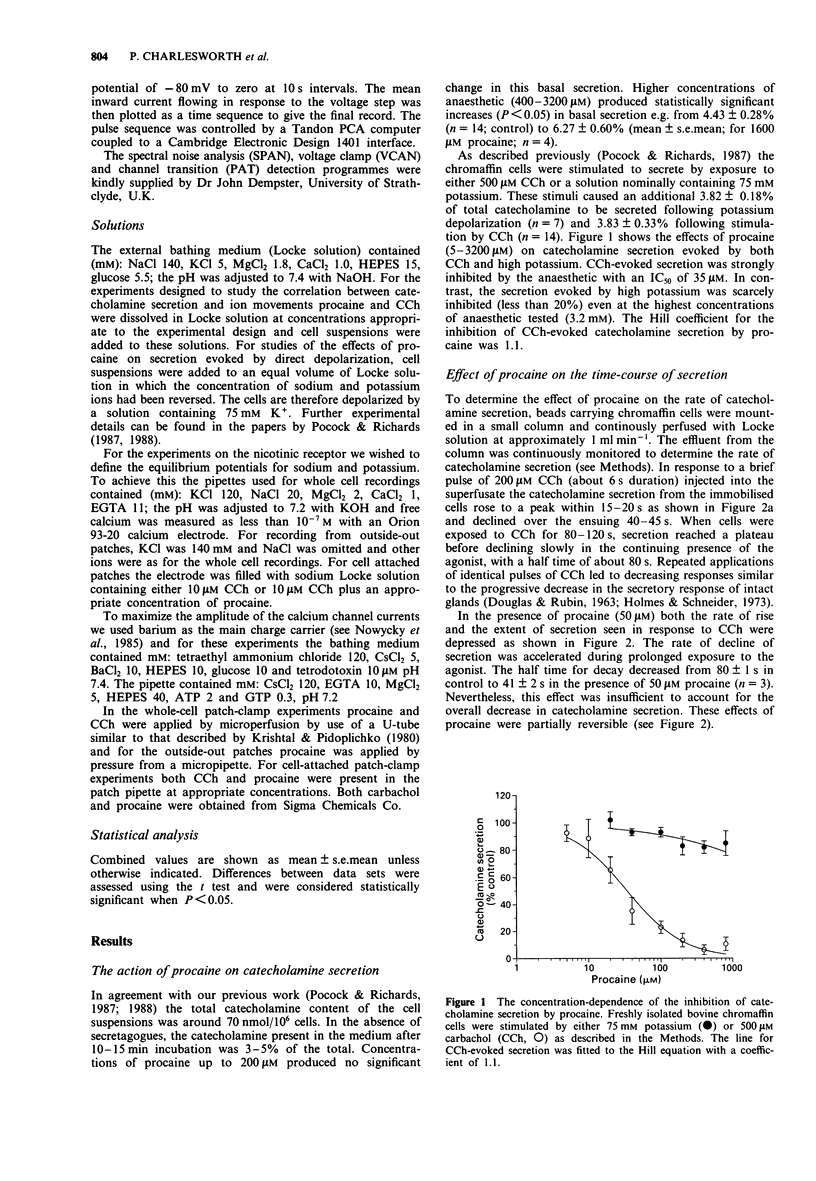
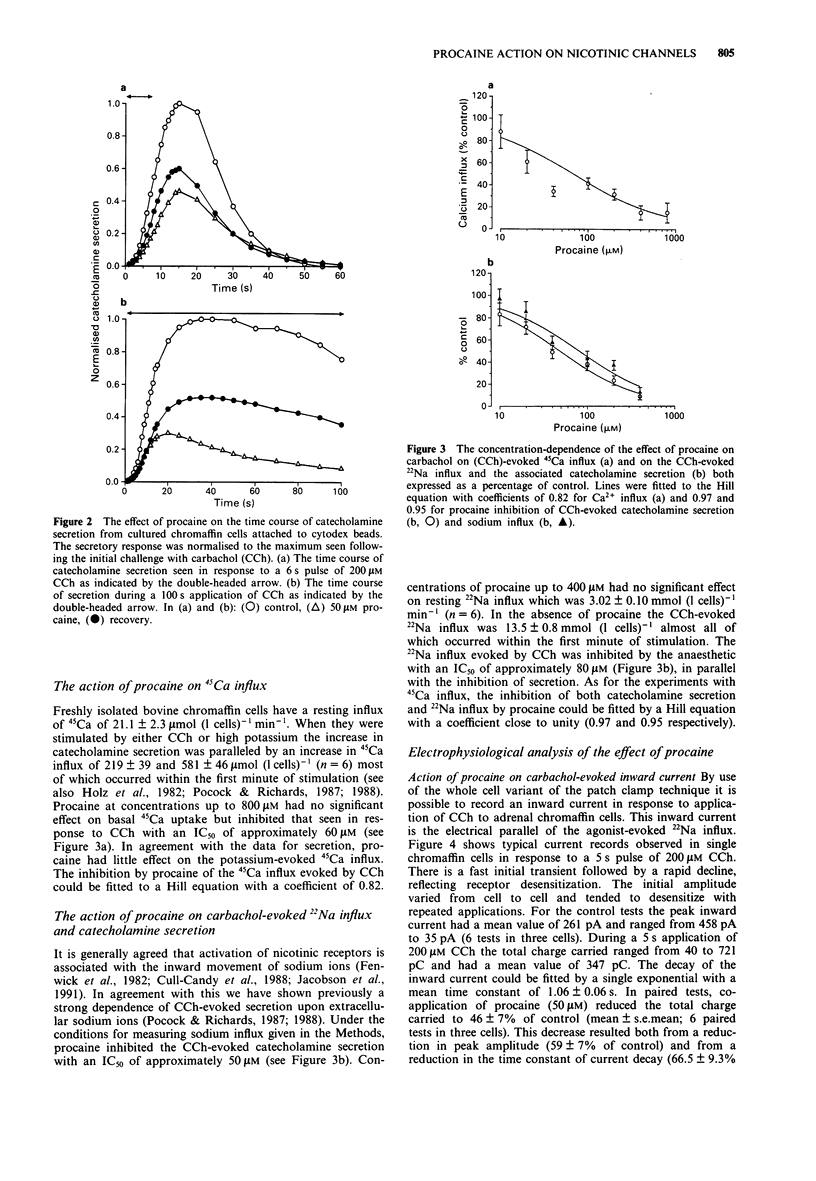
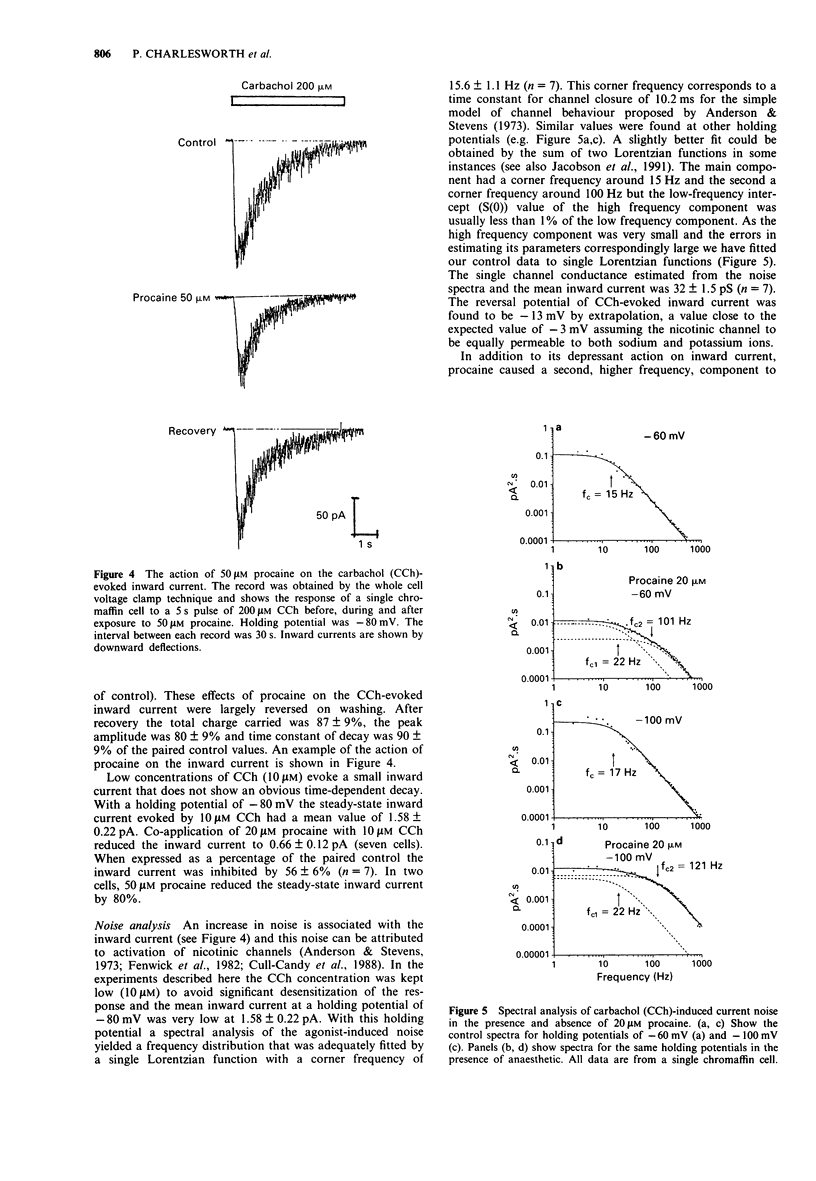
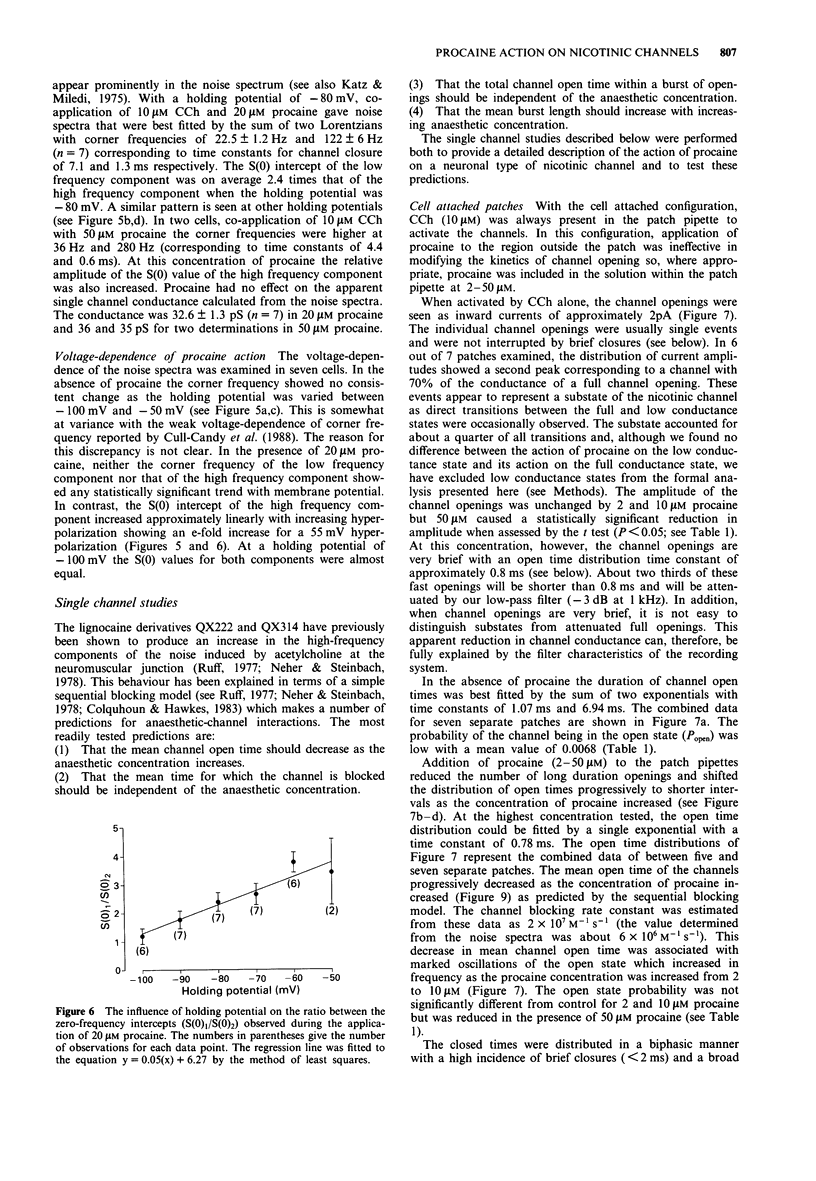
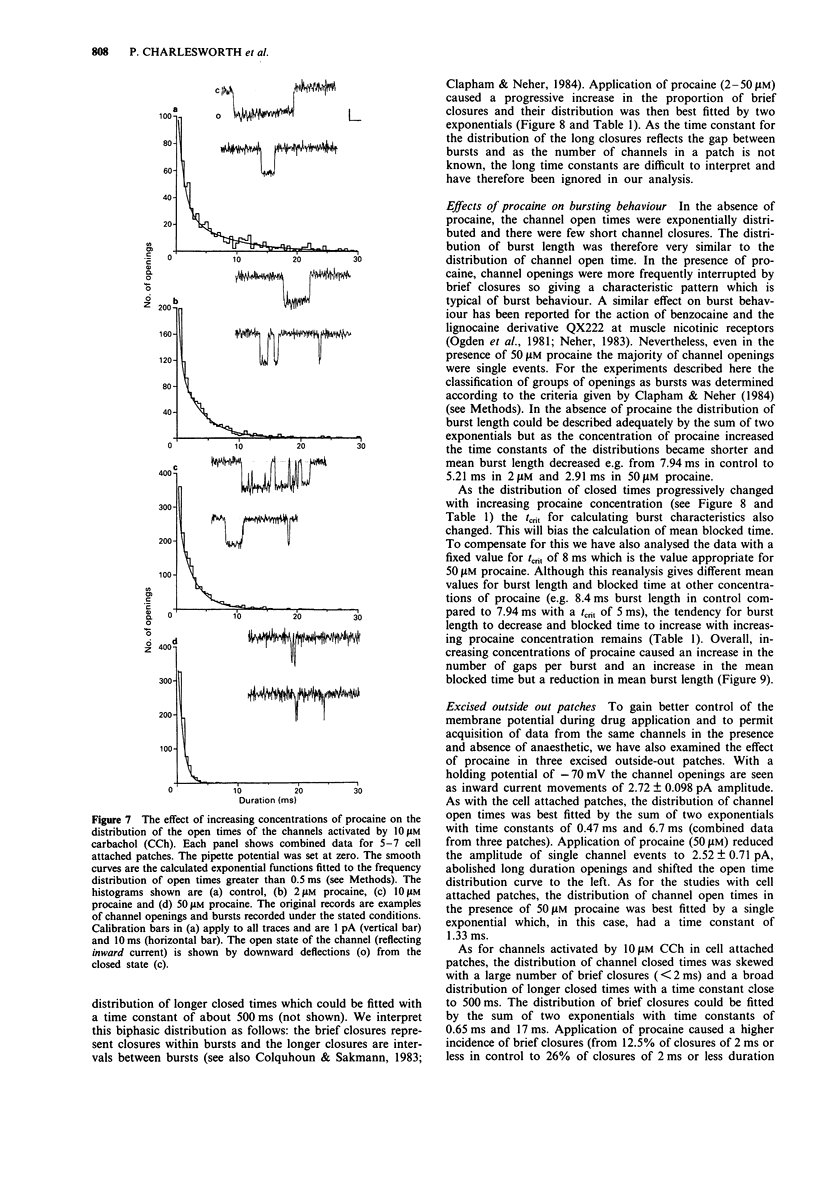
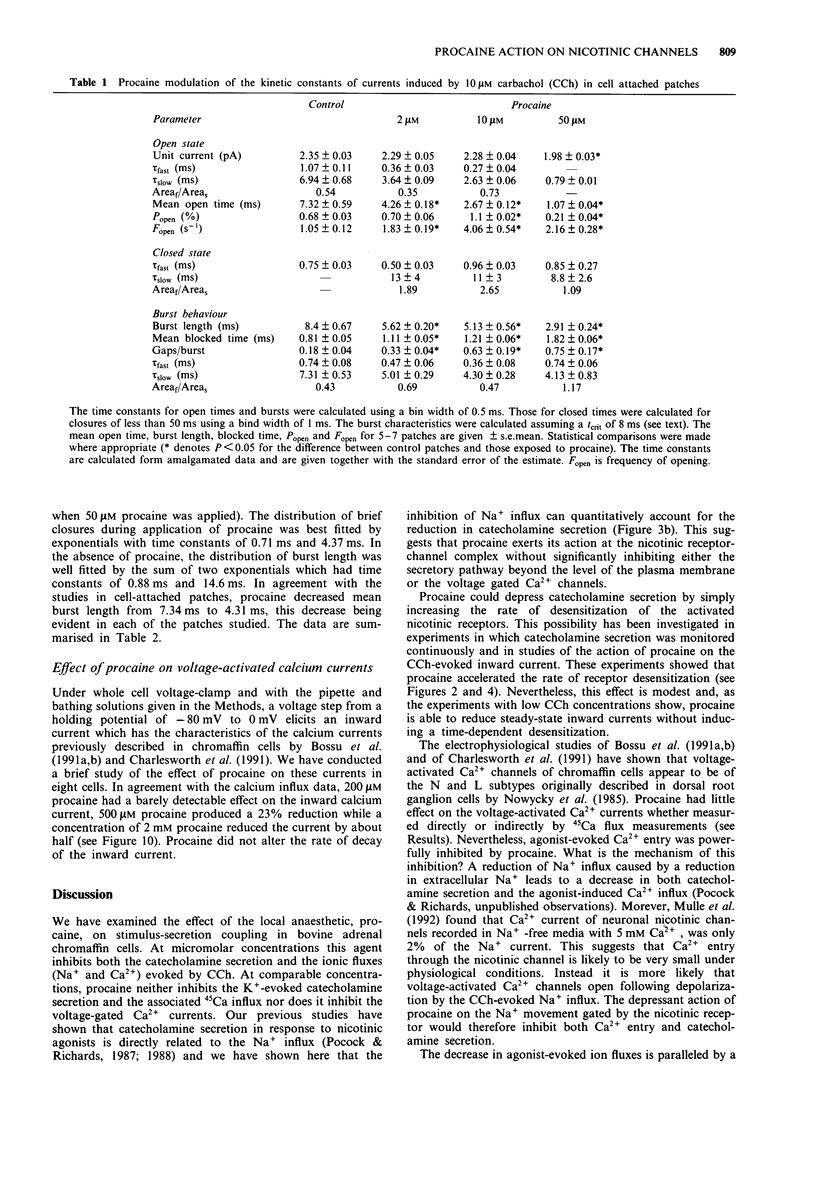
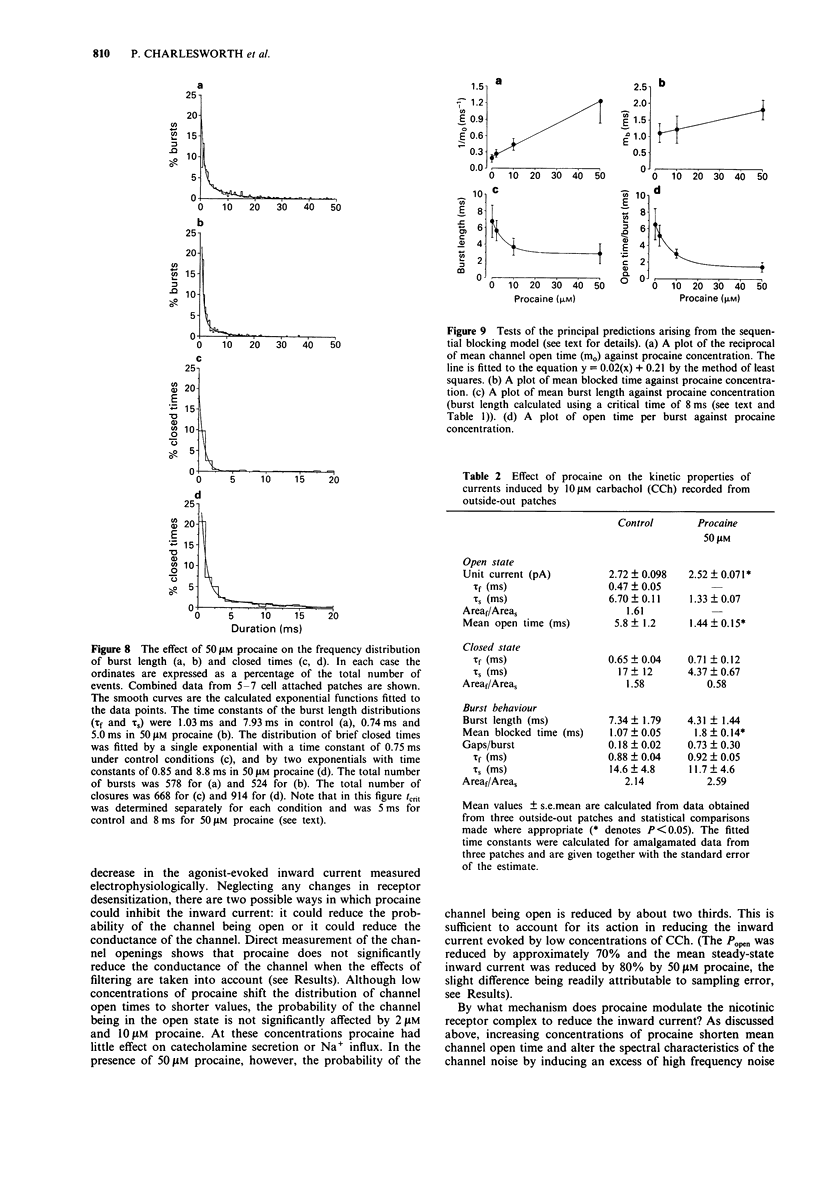
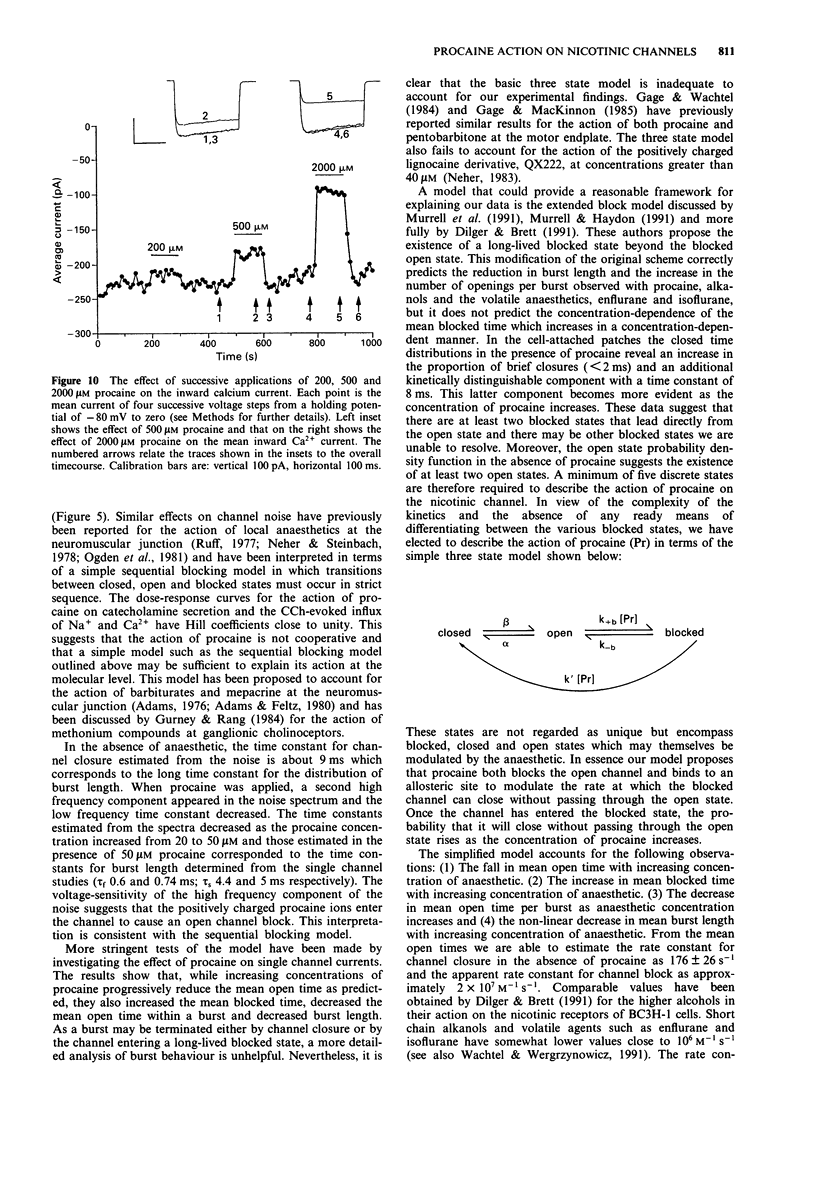
1. We have investigated the action of procaine on stimulus-secretion coupling in bovine adrenal chromaffin cells. 2. Procaine inhibited the catecholamine secretion evoked by 500 microM carbachol (CCh) with an IC50 of 35 microM and the associated calcium influx (IC50 60 microM). It inhibited the catecholamine secretion evoked by depolarization with high potassium by less than 20% even at the highest concentrations tested (3.2 mM). 3. The secretion evoked by CCh was associated with an increase in sodium influx. This evoked influx was also inhibited by procaine (IC50 80 microM). 4. This selective action of procaine on the CCh-evoked catecholamine secretion was investigated further by patch-clamp techniques. 5. In agreement with the ion flux studies, procaine inhibited the inward current evoked by CCh. Procaine also altered the spectral characteristics of the noise associated with the agonist-induced current by adding an additional high frequency component. The amplitude of this component showed an e-fold increase for a 55 mV membrane hyperpolarization. 6. Data from cell-attached patches showed that increasing concentrations of procaine produced a progressive fall in the mean channel open time and an increase in mean blocked time. This combination led to a decrease in mean burst length. In addition, Popen was reduced by 50 microM procaine. These changes in channel conducting time were sufficient to account for the reduction in inward current. A limited study of the action of procaine on nicotinic channels in outside-out patches gave similar results. 7. The data were considered in relation to various schemes of anaesthetic-channel interactions. The data did not fit the sequential blocking model or the extended channel block model but could be fitted to a modified sequential blocking model in which the rate constant for channel reopening after block was itself subject to modulation by the anaesthetic and the blocked channel could close without passing through the open state.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Feltz A. Quinacrine (mepacrine) action at frog end-plate. J Physiol. 1980 Sep;306:261–281. doi: 10.1113/jphysiol.1980.sp013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., De Waard M., Feltz A. Inactivation characteristics reveal two calcium currents in adult bovine chromaffin cells. J Physiol. 1991 Jun;437:603–620. doi: 10.1113/jphysiol.1991.sp018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., De Waard M., Feltz A. Two types of calcium channels are expressed in adult bovine chromaffin cells. J Physiol. 1991 Jun;437:621–634. doi: 10.1113/jphysiol.1991.sp018615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Mathie A., Powis D. A. Acetylcholine receptor channels and their block by clonidine in cultured bovine chromaffin cells. J Physiol. 1988 Aug;402:255–278. doi: 10.1113/jphysiol.1988.sp017203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger J. P., Brett R. S. Actions of volatile anesthetics and alcohols on cholinergic receptor channels. Ann N Y Acad Sci. 1991;625:616–627. doi: 10.1111/j.1749-6632.1991.tb33896.x. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McKinnon D. Effects of pentobarbitone on acetylcholine-activated channels in mammalian muscle. Br J Pharmacol. 1985 May;85(1):229–235. doi: 10.1111/j.1476-5381.1985.tb08851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Wachtel R. E. Some effects of procaine at the toad end-plate are not consistent with a simple channel-blocking model. J Physiol. 1984 Jan;346:331–339. doi: 10.1113/jphysiol.1984.sp015025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Rang H. P. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br J Pharmacol. 1984 Jul;82(3):623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A., Frye R. A. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem. 1982 Sep;39(3):635–646. doi: 10.1111/j.1471-4159.1982.tb07940.x. [DOI] [PubMed] [Google Scholar]
- Jacobson I., Pocock G., Richards C. D. Effects of pentobarbitone on the properties of nicotinic channels of chromaffin cells. Eur J Pharmacol. 1991 Sep 24;202(3):331–339. doi: 10.1016/0014-2999(91)90275-u. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of procaine on the action of acetylcholine at the neuromuscular junction. J Physiol. 1975 Jul;249(2):269–284. doi: 10.1113/jphysiol.1975.sp011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Marty A. Noise and relaxation studies of acetylcholine induced currents in the presence of procaine. J Physiol. 1978 May;278:237–250. doi: 10.1113/jphysiol.1978.sp012301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C., Choquet D., Korn H., Changeux J. P. Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron. 1992 Jan;8(1):135–143. doi: 10.1016/0896-6273(92)90115-t. [DOI] [PubMed] [Google Scholar]
- Murrell R. D., Braun M. S., Haydon D. A. Actions of n-alcohols on nicotinic acetylcholine receptor channels in cultured rat myotubes. J Physiol. 1991 Jun;437:431–448. doi: 10.1113/jphysiol.1991.sp018604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell R. D., Haydon D. A. Actions of n-alcohols on nicotinic acetylcholine receptor ion channels in cultured rat muscle cells. Ann N Y Acad Sci. 1991;625:365–374. doi: 10.1111/j.1749-6632.1991.tb33864.x. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Siegelbaum S. A., Colquhoun D. Block of acetylcholine-activated ion channels by an uncharged local anaesthetic. Nature. 1981 Feb 12;289(5798):596–598. doi: 10.1038/289596a0. [DOI] [PubMed] [Google Scholar]
- Pocock G. Ion movements in isolated bovine adrenal medullary cells treated with ouabain. Mol Pharmacol. 1983 May;23(3):681–697. [PubMed] [Google Scholar]
- Pocock G. Ionic and metabolic requirements for stimulation of secretion by ouabain in bovine adrenal medullary cells. Mol Pharmacol. 1983 May;23(3):671–680. [PubMed] [Google Scholar]
- Pocock G., Richards C. D. Cellular mechanisms in general anaesthesia. Br J Anaesth. 1991 Jan;66(1):116–128. doi: 10.1093/bja/66.1.116. [DOI] [PubMed] [Google Scholar]
- Pocock G., Richards C. D. The action of pentobarbitone on stimulus-secretion coupling in adrenal chromaffin cells. Br J Pharmacol. 1987 Jan;90(1):71–80. doi: 10.1111/j.1476-5381.1987.tb16826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock G., Richards C. D. The action of volatile anaesthetics on stimulus-secretion coupling in bovine adrenal chromaffin cells. Br J Pharmacol. 1988 Sep;95(1):209–217. doi: 10.1111/j.1476-5381.1988.tb16566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D. The actions of pentobarbitone, procaine and tetrodotoxin on synaptic transmission in the olfactory cortex of the guinea-pig. Br J Pharmacol. 1982 Apr;75(4):639–646. doi: 10.1111/j.1476-5381.1982.tb09185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- Wachtel R. E., Wegrzynowicz E. S. Mechanism of volatile anesthetic action on ion channels. Ann N Y Acad Sci. 1991;625:116–128. doi: 10.1111/j.1749-6632.1991.tb33835.x. [DOI] [PubMed] [Google Scholar]
- Waymire J. C., Bennett W. F., Boehme R., Hankins L., Gilmer-Waymire K., Haycock J. W. Bovine adrenal chromaffin cells: high-yield purification and viability in suspension culture. J Neurosci Methods. 1983 Apr;7(4):329–351. doi: 10.1016/0165-0270(83)90026-2. [DOI] [PubMed] [Google Scholar]


