Abstract
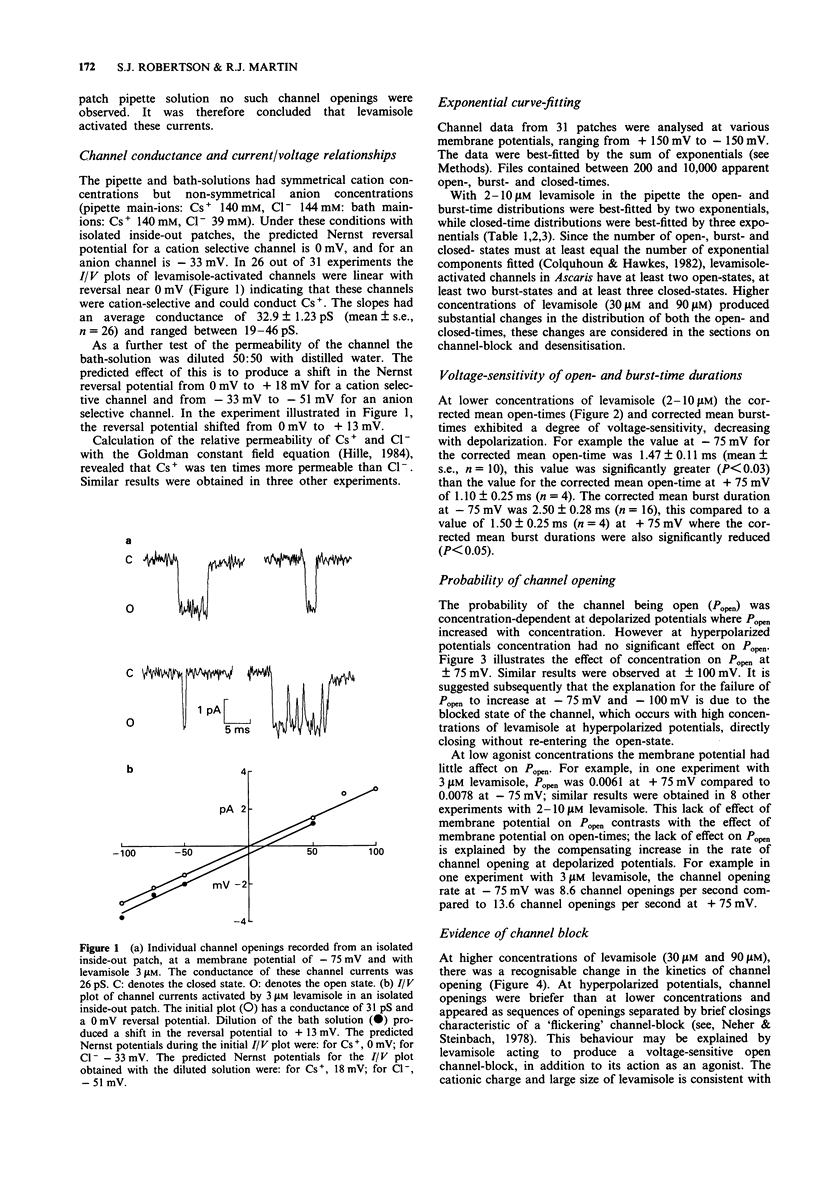
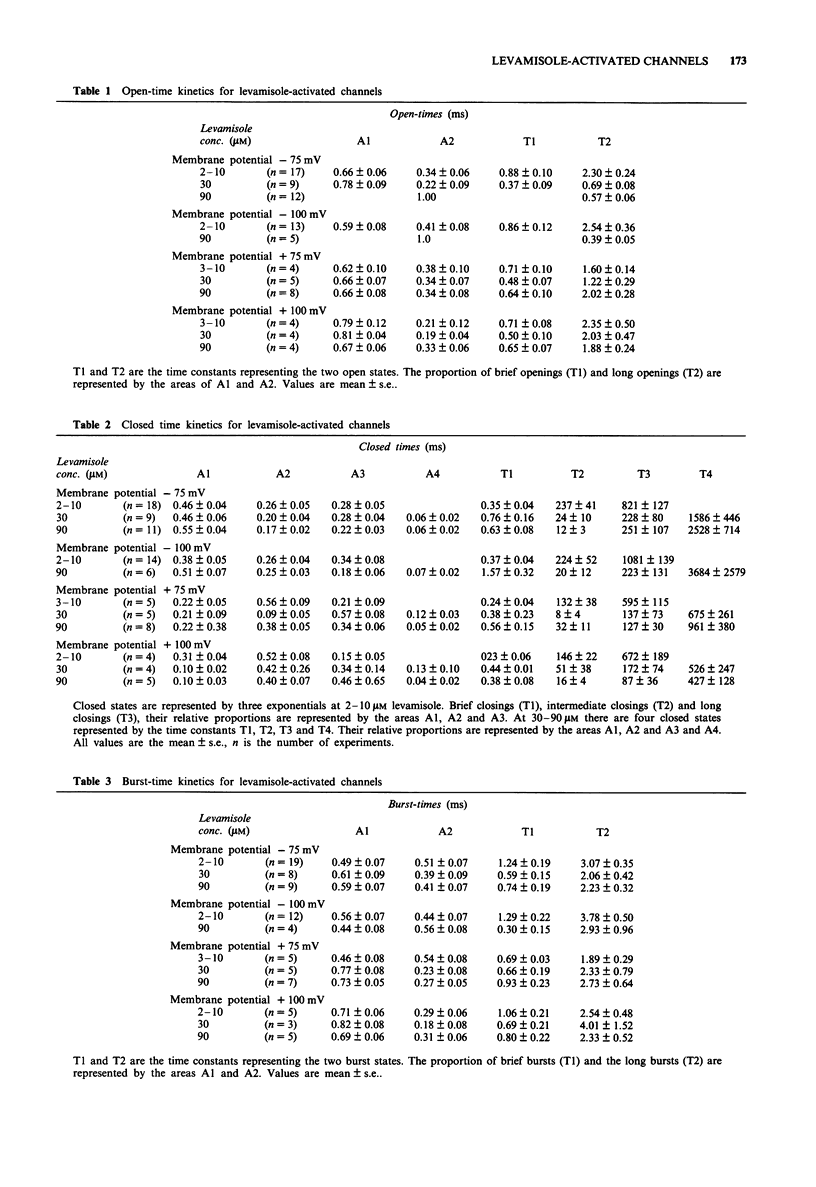
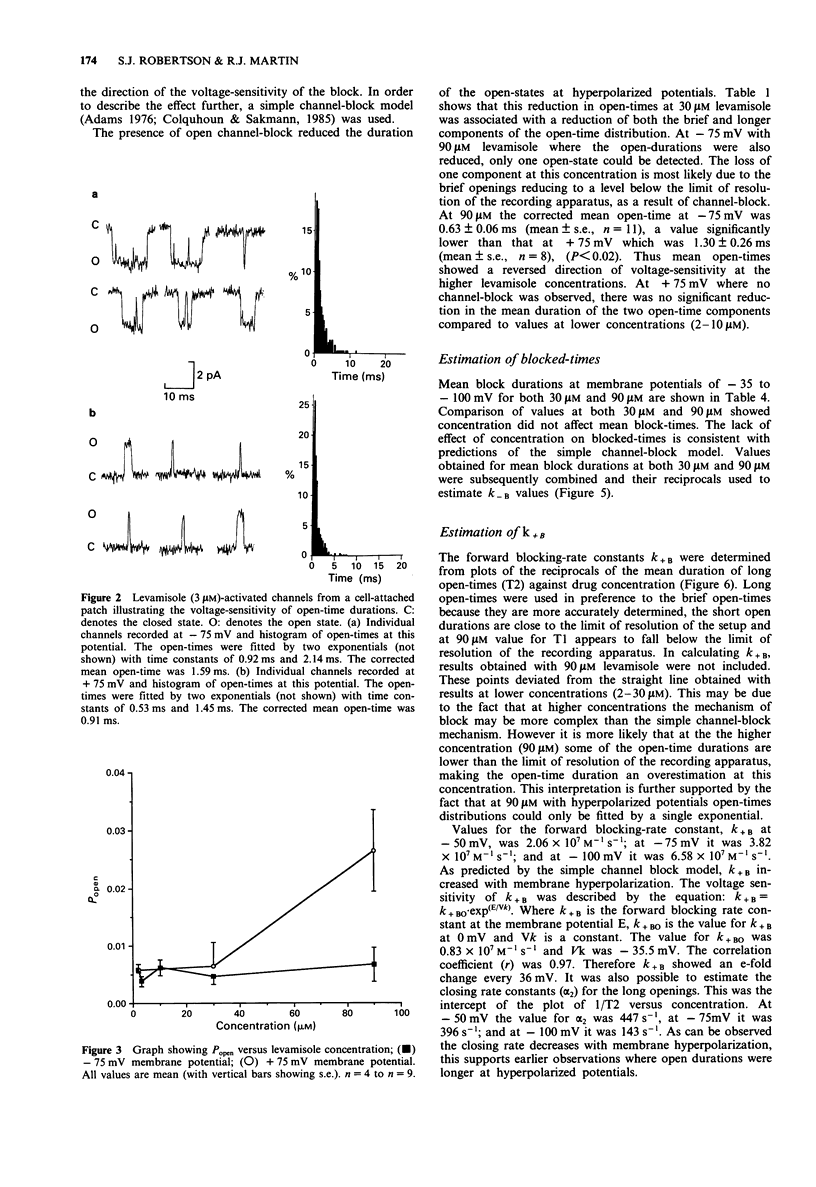
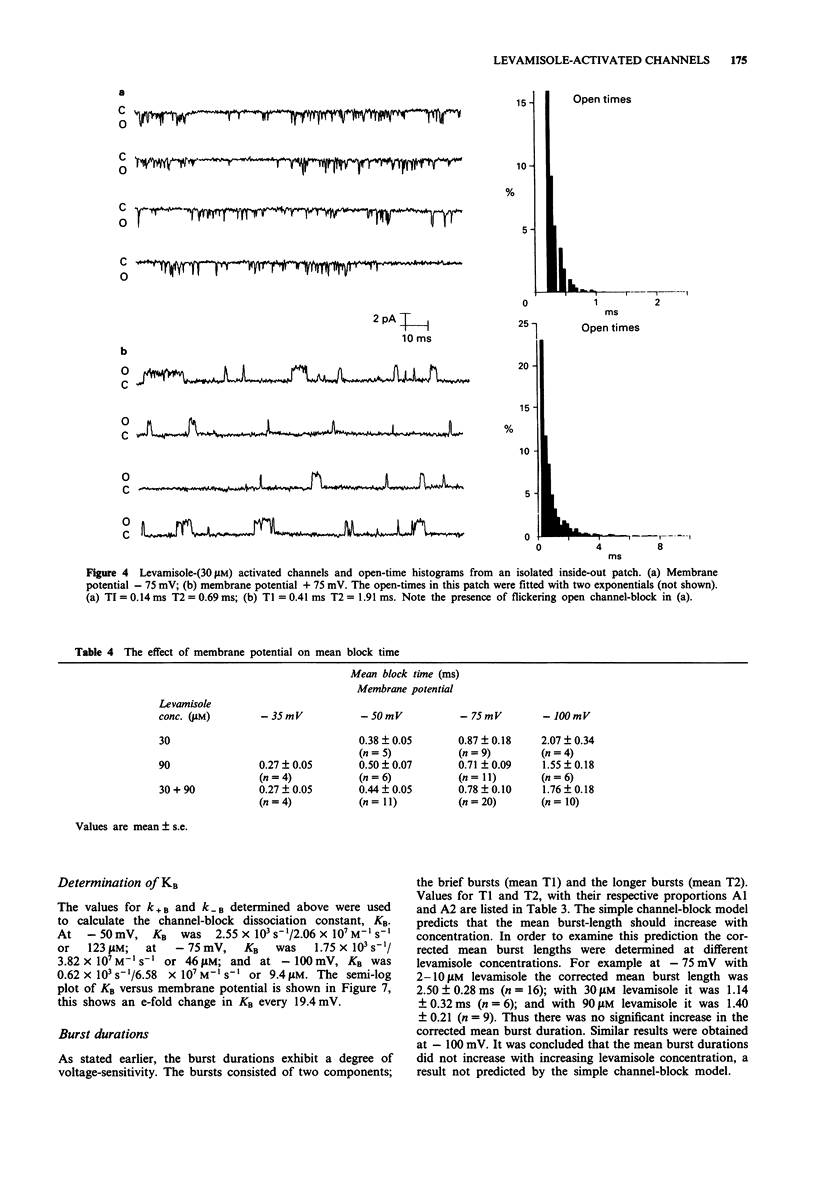
1. The patch-clamp technique was used to examine levamisole-activated channels in muscle vesicles from Ascaris suum. Cell-attached and isolated inside-out patches were used. 2. Levamisole (1-90 microM), applied to the extracellular surface, activated channels which had apparent mean open-times in the range 0.80-2.85 ms and linear I/V relationships with conductances in the range 19-46 pS. Ion-replacement experiments showed the channels to be cation selective. 3. The kinetics of the channels were analysed. Generally open- and closed-time distributions were best fitted by two, and three exponentials respectively, indicating the presence of at least two open states and at least three closed states. The distributions of burst-times were best-fitted by two exponentials. 4. Channel open- and burst-times were voltage-sensitive: at low levamisole concentrations (1-10 microM), they increased with hyperpolarization. At higher concentrations of levamisole (30 microM and 90 microM) flickering channel-block was observed at hyperpolarized potentials. Using a simple channel-block model, values for the blocking dissociation constant, KB were determined as 123 microM at -50 mV, 46 microM at -75 mV and 9.4 microM at -100 mV. 5. At the higher concentration of levamisole (30 microM and 90 microM) long closed-times separating 'clusters' of bursts were observed, at both hyperpolarized and depolarized membrane potentials and this was interpreted as desensitization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceves J., Erlij D., Martínez-Marañn R. The mechanism of the paralysing action of tetramisole on Ascaris somatic muscle. Br J Pharmacol. 1970 May;38(3):602–607. doi: 10.1111/j.1476-5381.1970.tb10601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., Colquhoun D. Desensitization of the acetylcholine receptor of frog end-plates measured in a Vaseline-gap voltage clamp. J Physiol. 1989 Aug;415:159–188. doi: 10.1113/jphysiol.1989.sp017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles G. C., East J. M., Jenkins S. M. The mode of action of four anthelmintics. Experientia. 1974 Nov 15;30(11):1265–1266. doi: 10.1007/BF01945176. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun L., Holden-Dye L., Walker R. J. The pharmacology of cholinoceptors on the somatic muscle cells of the parasitic nematode Ascaris suum. J Exp Biol. 1991 Jul;158:509–530. doi: 10.1242/jeb.158.1.509. [DOI] [PubMed] [Google Scholar]
- Martin R. J. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. Br J Pharmacol. 1982 Oct;77(2):255–265. doi: 10.1111/j.1476-5381.1982.tb09294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J., Kusel J. R., Pennington A. J. Surface properties of membrane vesicles prepared from muscle cells of Ascaris suum. J Parasitol. 1990 Jun;76(3):340–348. [PubMed] [Google Scholar]
- Martin R. J. gamma-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br J Pharmacol. 1985 Feb;84(2):445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoff I. L. The pharmacology of the cholinoceptor in muscle preparations of Ascaris lumbricoides var. suum. Br J Pharmacol. 1969 Sep;37(1):251–257. doi: 10.1111/j.1476-5381.1969.tb09542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington A. J., Martin R. J. A patch-clamp study of acetylcholine-activated ion channels in Ascaris suum muscle. J Exp Biol. 1990 Nov;154:201–221. doi: 10.1242/jeb.154.1.201. [DOI] [PubMed] [Google Scholar]
- Rozhkova E. K., Malyutina T. A., Shishov B. A. Pharmacological characteristics of cholinoreception in somatic muscles of the nematode, Ascaris suum. Gen Pharmacol. 1980;11(1):141–146. doi: 10.1016/0306-3623(80)90023-3. [DOI] [PubMed] [Google Scholar]
- Standen O. D. Chemotherapy of intestinal helminthiasis. Prog Drug Res. 1975;19:158–165. doi: 10.1007/978-3-0348-7090-0_20. [DOI] [PubMed] [Google Scholar]
- Thorn P., Martin R. J. A high-conductance calcium-dependent chloride channel in Ascaris suum muscle. Q J Exp Physiol. 1987 Jan;72(1):31–49. doi: 10.1113/expphysiol.1987.sp003053. [DOI] [PubMed] [Google Scholar]


