Abstract
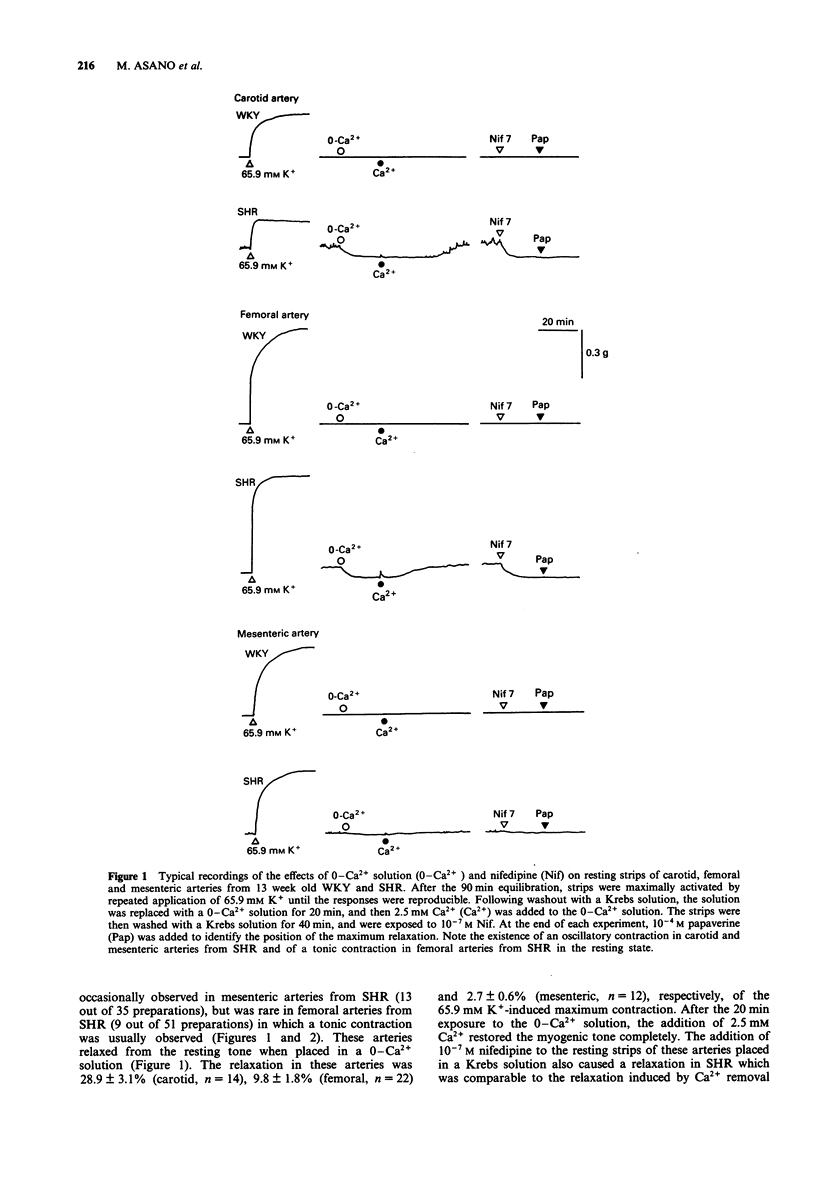
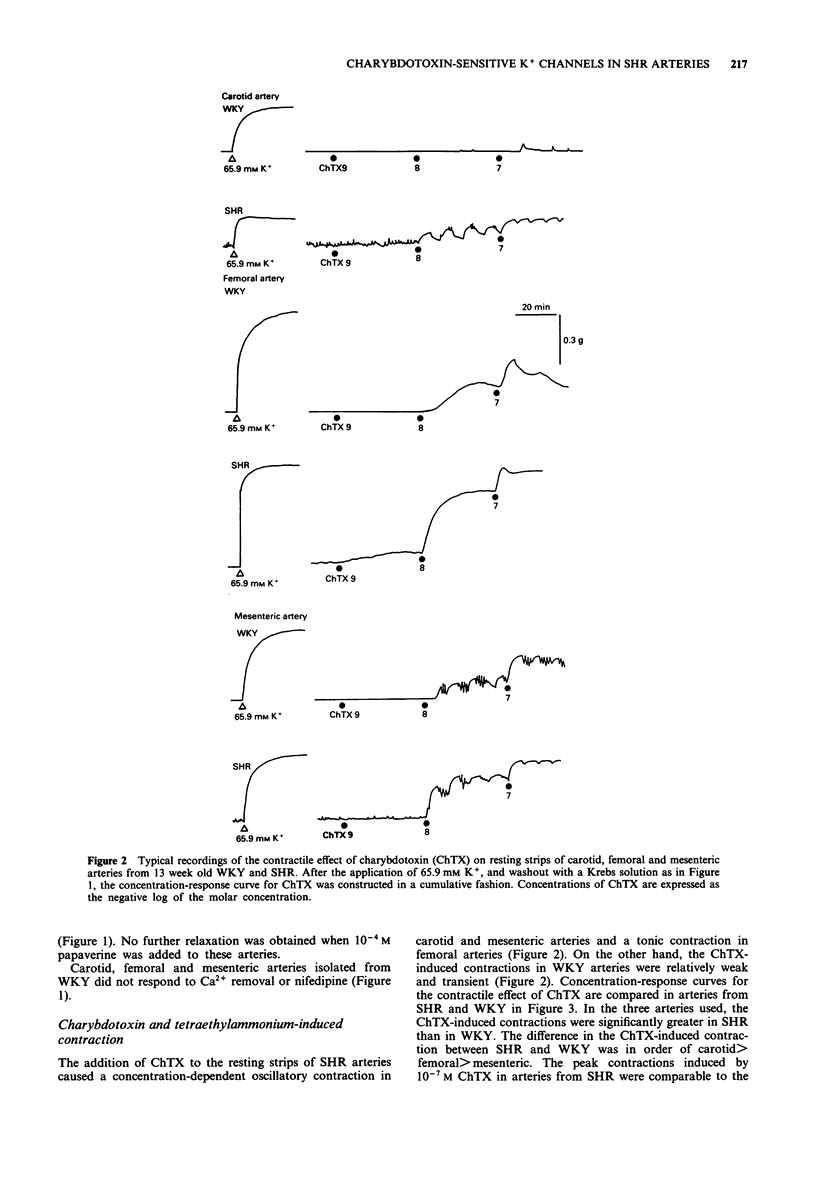
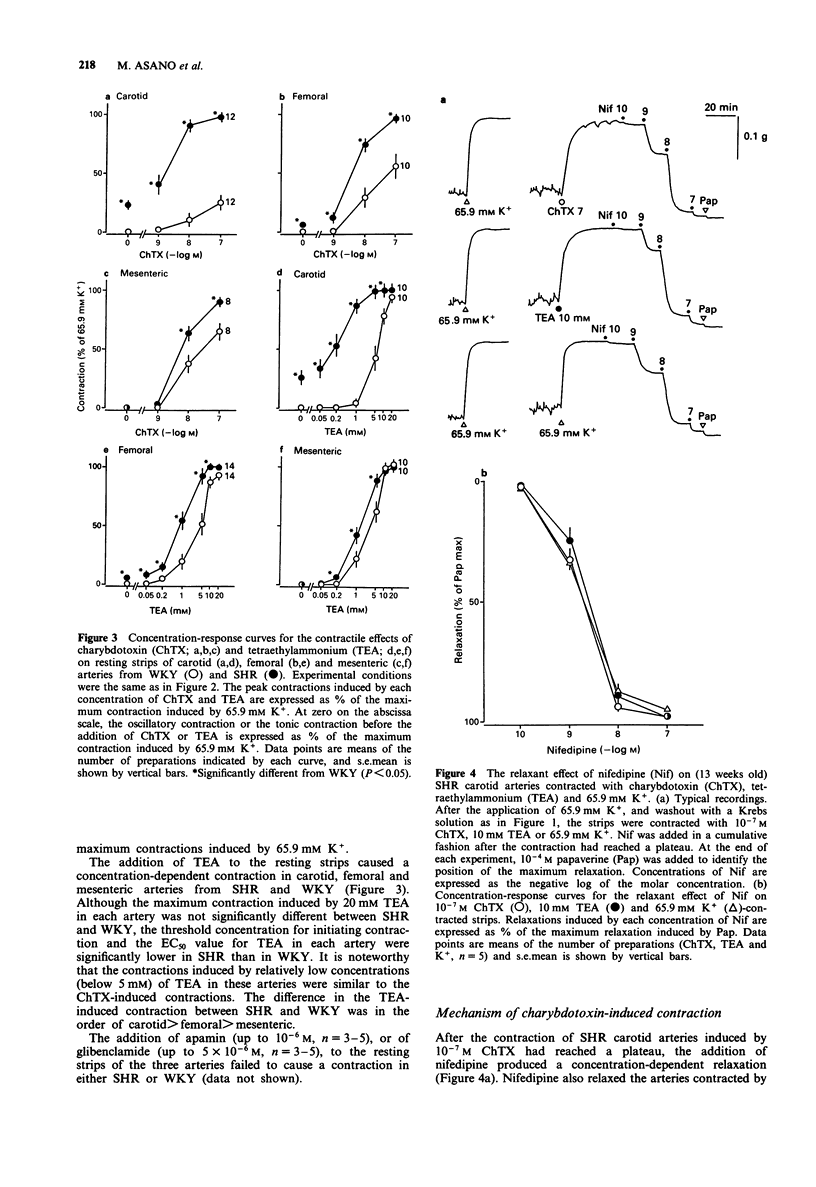
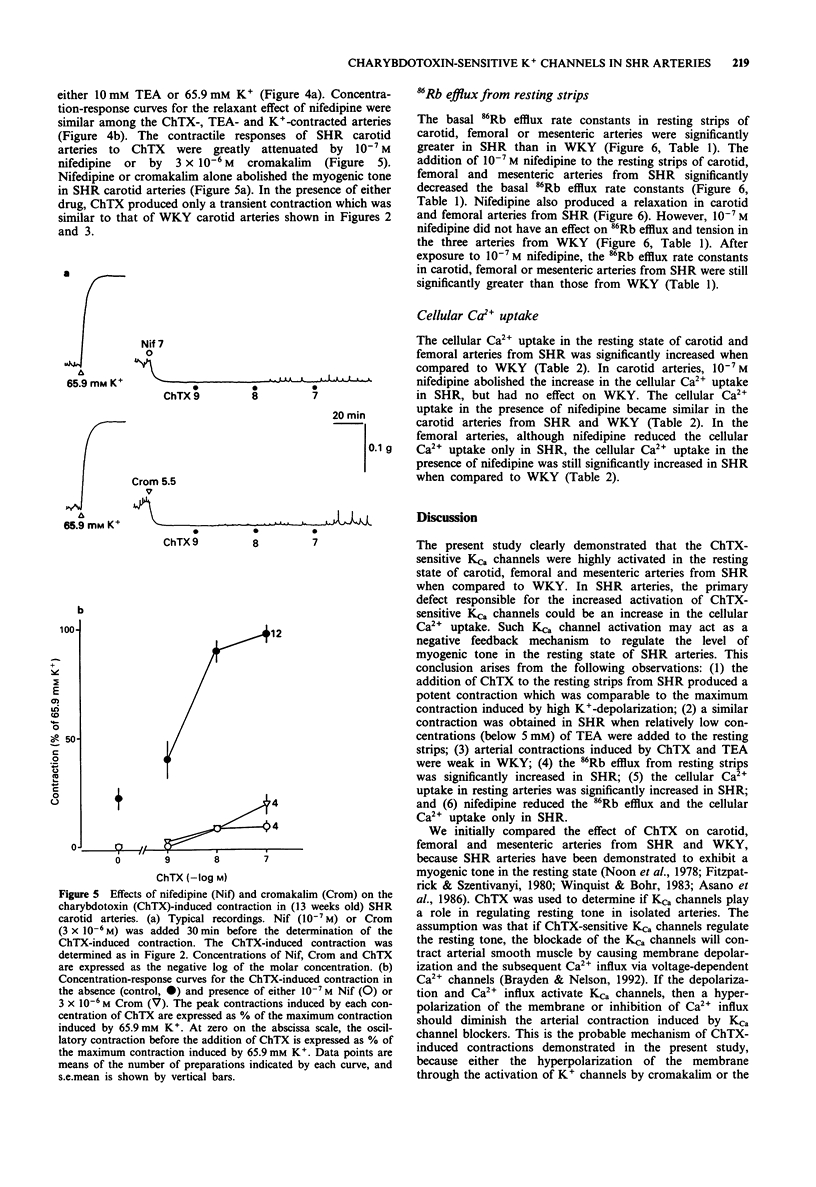
1. To determine the possible role of Ca(2+)-activated K+ (KCa) channels in the regulation of resting tone of arteries from spontaneously hypertensive rats (SHR), the effects of agents which interact with these channels on tension and 86Rb efflux were compared in endothelium-denuded strips of carotid, femoral and mesenteric arteries from SHR and normotensive Wistar-Kyoto rats (WKY). 2. Strips of carotid, femoral and mesenteric arteries from SHR exhibited a myogenic tone; that is, the resting tone decreased when either the Krebs solution was changed to a 0-Ca2+ solution or 10(-7) M nifedipine was added. 3. The addition of charybdotoxin (ChTX, 10(-9)-10(-7) M), a blocker of large conductance KCa channels, to the resting strips of these arteries produced a concentration-dependent contraction, which was significantly greater in SHR than in WKY. Relatively low concentrations of tetraethylammonium (0.05-5 mM) produced a concentration-dependent contraction which was similar to the ChTX-induced contraction in these strips. 4. The ChTX-induced contractions in SHR were greatly attenuated by 10(-7) M nifedipine and by 3 x 10(-6) M cromakalim, a K+ channel opener. Cromakalim alone abolished the myogenic tone in SHR. 5. The addition of apamin (a blocker of small conductance KCa channels, up to 10(-6) M), or of glibenclamide (a blocker of ATP-sensitive K+ channels, up to 5 x 10(-6) M), to the resting strips failed to produce a contraction. 6. In resting strips of carotid, femoral and mesenteric arteries preloaded with 86Rb, the basal 86Rb efflux rate constants were significantly greater in SHR than in WKY.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano M., Aoki K., Matsuda T. Contractile effects of Bay k 8644, a dihydropyridine calcium agonist, on isolated femoral arteries from spontaneously hypertensive rats. J Pharmacol Exp Ther. 1986 Oct;239(1):198–205. [PubMed] [Google Scholar]
- Asano M., Hidaka H. Pharmacological properties of N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W-7), a calmodulin antagonist in arterial strips from rats and rabbits. J Pharmacol Exp Ther. 1985 Aug;234(2):476–484. [PubMed] [Google Scholar]
- Asano M., Masuzawa K., Matsuda T. Evidence for reduced beta-adrenoceptor coupling to adenylate cyclase in femoral arteries from spontaneously hypertensive rats. Br J Pharmacol. 1988 May;94(1):73–86. doi: 10.1111/j.1476-5381.1988.tb11501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr D. F., Webb R. C. Vascular smooth muscle membrane in hypertension. Annu Rev Pharmacol Toxicol. 1988;28:389–409. doi: 10.1146/annurev.pa.28.040188.002133. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G., Jenkinson D. H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989 Feb;12(2):59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Cheung D. W. Membrane potential of vascular smooth muscle and hypertension in spontaneously hypertensive rats. Can J Physiol Pharmacol. 1984 Aug;62(8):957–960. doi: 10.1139/y84-160. [DOI] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. F., Szentivanyi A. The relationship between increased myogenic tone and hyporesponsiveness in vascular smooth muscle of spontaneously hypertensive rats. Clin Exp Hypertens. 1980;2(6):1023–1037. doi: 10.3109/10641968009037159. [DOI] [PubMed] [Google Scholar]
- Fujii K., Tominaga M., Ohmori S., Kobayashi K., Koga T., Takata Y., Fujishima M. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res. 1992 Apr;70(4):660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Jelicks L. A., Gupta R. K. NMR measurement of cytosolic free calcium, free magnesium, and intracellular sodium in the aorta of the normal and spontaneously hypertensive rat. J Biol Chem. 1990 Jan 25;265(3):1394–1400. [PubMed] [Google Scholar]
- Jones A. W. Altered ion transport in vascular smooth muscle from spontaneously hypertensive rats. Influences of aldosterone, norepinephrine, and angiotensin. Circ Res. 1973 Nov;33(5):563–572. doi: 10.1161/01.res.33.5.563. [DOI] [PubMed] [Google Scholar]
- Jones A. W. Reactivity of ion fluxes in rat aorta during hypertension and circulatory control. Fed Proc. 1974 Feb;33(2):133–137. [PubMed] [Google Scholar]
- Lau K., Eby B. The role of calcium in genetic hypertension. Hypertension. 1985 Sep-Oct;7(5):657–667. doi: 10.1161/01.hyp.7.5.657. [DOI] [PubMed] [Google Scholar]
- Masuzawa K., Asano M., Matsuda T., Imaizumi Y., Watanabe M. Comparison of effects of cromakalim and pinacidil on mechanical activity and 86Rb efflux in dog coronary arteries. J Pharmacol Exp Ther. 1990 May;253(2):586–593. [PubMed] [Google Scholar]
- Masuzawa K., Matsuda T., Asano M. The diverse effects of cromakalim on tension and 86Rb efflux in canine arterial smooth muscle. Br J Pharmacol. 1991 May;103(1):1033–1040. doi: 10.1111/j.1476-5381.1991.tb12296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Noon J. P., Rice P. J., Baldessarini R. J. Calcium leakage as a cause of the high resting tension in vascular smooth muscle from the spontaneously hypertensive rat. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1605–1607. doi: 10.1073/pnas.75.3.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavenstädt H., Lindeman S., Lindeman V., Späth M., Kunzelmann K., Greger R. Potassium conductance of smooth muscle cells from rabbit aorta in primary culture. Pflugers Arch. 1991 Aug;419(1):57–68. doi: 10.1007/BF00373748. [DOI] [PubMed] [Google Scholar]
- Postnov Y. V., Orlov S. N. Ion transport across plasma membrane in primary hypertension. Physiol Rev. 1985 Oct;65(4):904–945. doi: 10.1152/physrev.1985.65.4.904. [DOI] [PubMed] [Google Scholar]
- Sada T., Koike H., Ikeda M., Sato K., Ozaki H., Karaki H. Cytosolic free calcium of aorta in hypertensive rats. Chronic inhibition of angiotensin converting enzyme. Hypertension. 1990 Sep;16(3):245–251. doi: 10.1161/01.hyp.16.3.245. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Jones A. W. Calcium antagonists inhibit elevated potassium efflux from aorta of aldosterone-salt hypertensive rats. Hypertension. 1990 Jan;15(1):78–83. doi: 10.1161/01.hyp.15.1.78. [DOI] [PubMed] [Google Scholar]
- Sugg E. E., Garcia M. L., Reuben J. P., Patchett A. A., Kaczorowski G. J. Synthesis and structural characterization of charybdotoxin, a potent peptidyl inhibitor of the high conductance Ca2(+)-activated K+ channel. J Biol Chem. 1990 Nov 5;265(31):18745–18748. [PubMed] [Google Scholar]
- Thompson L. P., Bruner C. A., Lamb F. S., King C. M., Webb R. C. Calcium influx and vascular reactivity in systemic hypertension. Am J Cardiol. 1987 Jan 23;59(2):29A–34A. doi: 10.1016/0002-9149(87)90173-1. [DOI] [PubMed] [Google Scholar]
- Tomobe Y., Ishikawa T., Yanagisawa M., Kimura S., Masaki T., Goto K. Mechanisms of altered sensitivity to endothelin-1 between aortic smooth muscles of spontaneously hypertensive and Wistar-Kyoto rats. J Pharmacol Exp Ther. 1991 May;257(2):555–561. [PubMed] [Google Scholar]
- Van Breemen C., Cauvin C., Johns A., Leijten P., Yamamoto H. Ca2+ regulation of vascular smooth muscle. Fed Proc. 1986 Nov;45(12):2746–2751. [PubMed] [Google Scholar]
- Winquist R. J., Bohr D. F. Structural and functional changes in cerebral arteries from spontaneously hypertensive rats. Hypertension. 1983 May-Jun;5(3):292–297. doi: 10.1161/01.hyp.5.3.292. [DOI] [PubMed] [Google Scholar]



