Abstract
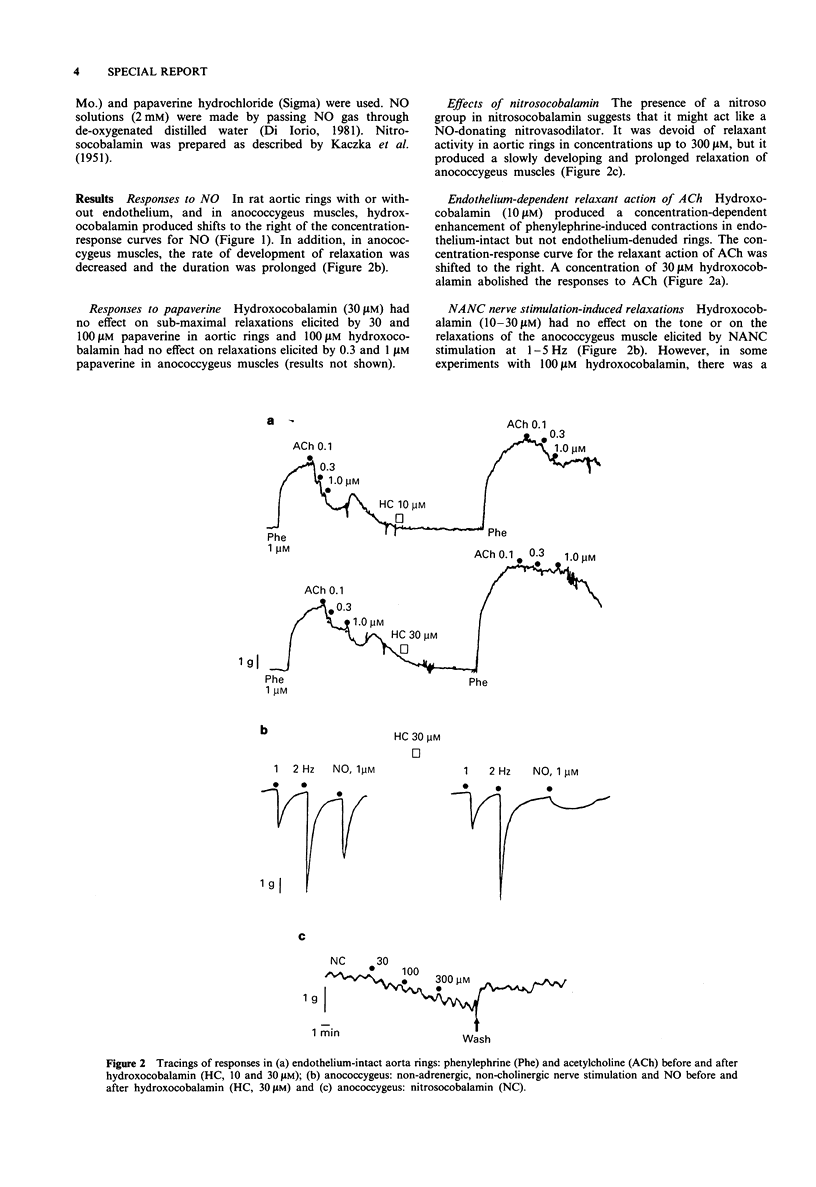
In rat aortic rings, hydroxocobalamin (10-30 microM) produced concentration-dependent reductions of the relaxant action of nitric oxide (NO) and the endothelium-dependent, NO-mediated, relaxant action of acetylcholine. In anococcygeus muscles, hydroxocobalamin (10-30 microM) reduced but also prolonged, NO-induced relaxations, but had no effect on non-adrenergic, non-cholinergic-mediated relaxations. Hydroxocobalamin had no effect on the NO-independent relaxant action of papaverine in either tissue. It is suggested that hydroxocobalamin sequesters NO by forming nitrosocobalamin. Nitrosocobalamin did not relax aortic rings, but produced a slowly developing and prolonged relaxation of anococcygeus muscles.
Full text
PDF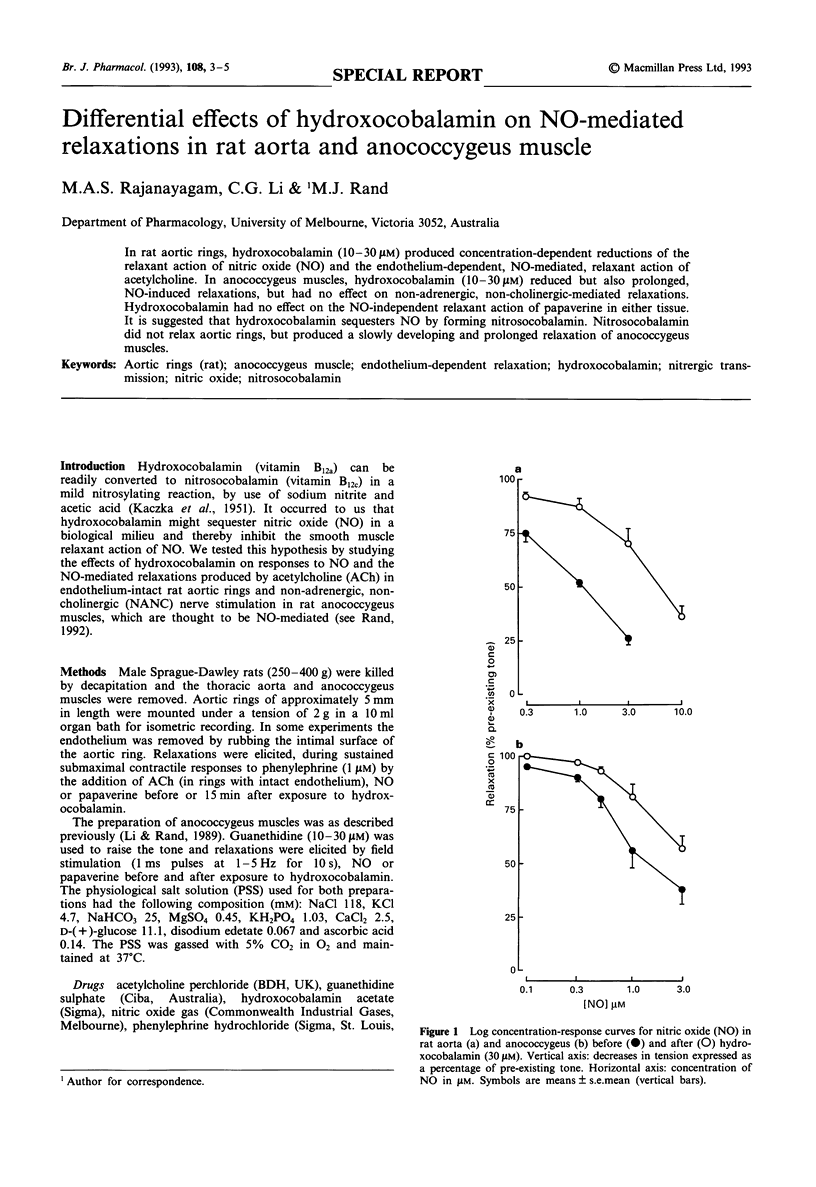

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Di Iorio E. E. Preparation of derivatives of ferrous and ferric hemoglobin. Methods Enzymol. 1981;76:57–72. doi: 10.1016/0076-6879(81)76114-7. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. A comparison of haemoglobin and erythrocytes as inhibitors of smooth muscle relaxation by the NANC transmitter in the BRP and rat anococcygeus and by EDRF in the rabbit aortic strip. Br J Pharmacol. 1989 Oct;98(2):445–450. doi: 10.1111/j.1476-5381.1989.tb12616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Khan M. T., Jothianandan D., Matsunaga K., Furchgott R. F. Vasodilation induced by acetylcholine and by glyceryl trinitrate in rat aortic and mesenteric vasculature. J Vasc Res. 1992 Jan-Feb;29(1):20–28. doi: 10.1159/000158927. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]


