Abstract
1. Patch clamp techniques were used to measure the ionic dependence of amiloride block of single mechanosensitive (MS) channels in frog (Xenopus laevis) oocytes. 2. The primary aim was to determine whether the difference in potency of amiloride block of MS channels in frog oocytes (IC50 = 0.5 mM) and chick auditory hair cells (IC50 = 50 microM) was due to the different ionic recording solutions. 3. Amiloride block of the oocyte MS channel does not vary significantly with complete substitution of external Na+ (i.e. 100 mM) with K+ in Ca(2+)-free recording solution (in both Na+ and K+ the IC50 = 0.5 mM). 4. A physiological concentration (1.8 mM) of external Ca2+ blocks the oocyte MS channel and reduces the potency of amiloride block (IC50 = 1.1 mM) without altering the voltage-dependence or the HIll coefficient (n = 1.8) of amiloride block. The reduction in potency can be explained by surface charge screening by Ca2+ which reduces the effective amiloride surface concentration. 5. The present results indicate that factors other than ionic recording conditions must underlie the difference in potency of amiloride block of MS channels in oocytes and auditory hair cells.
Full text
PDF
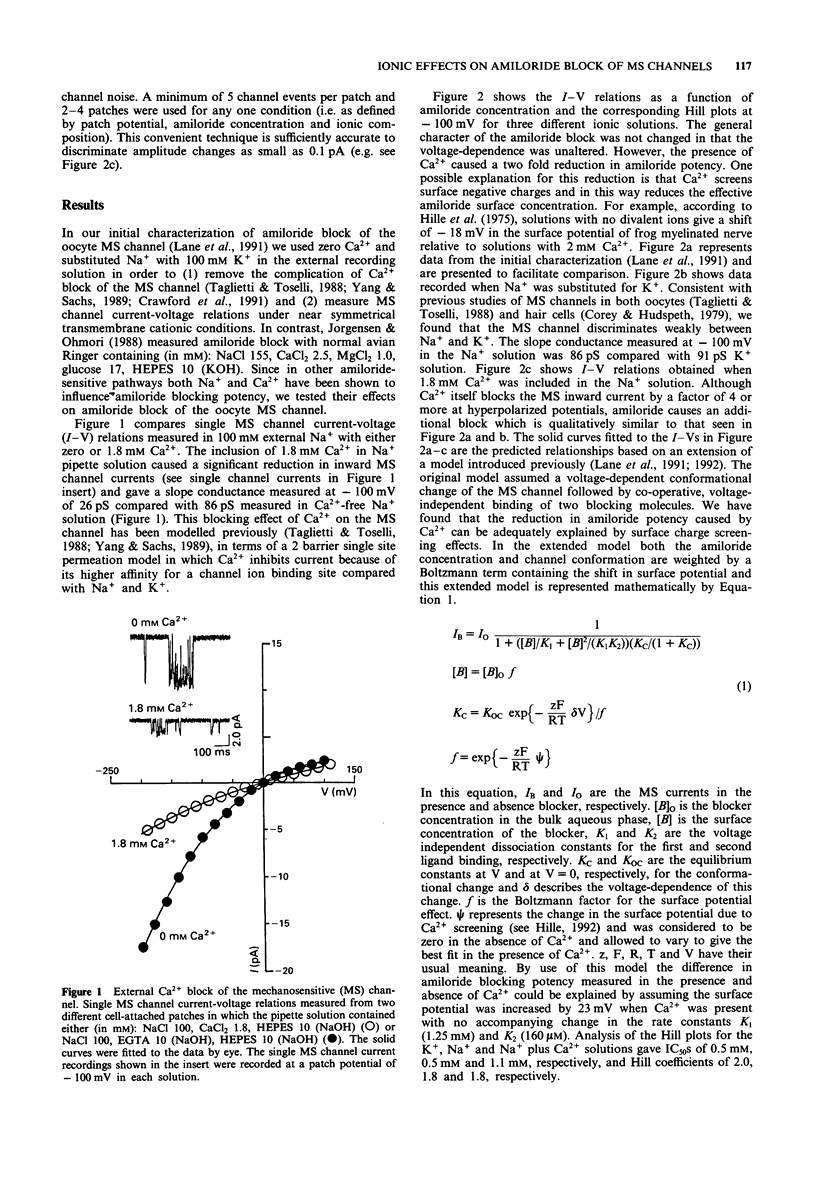
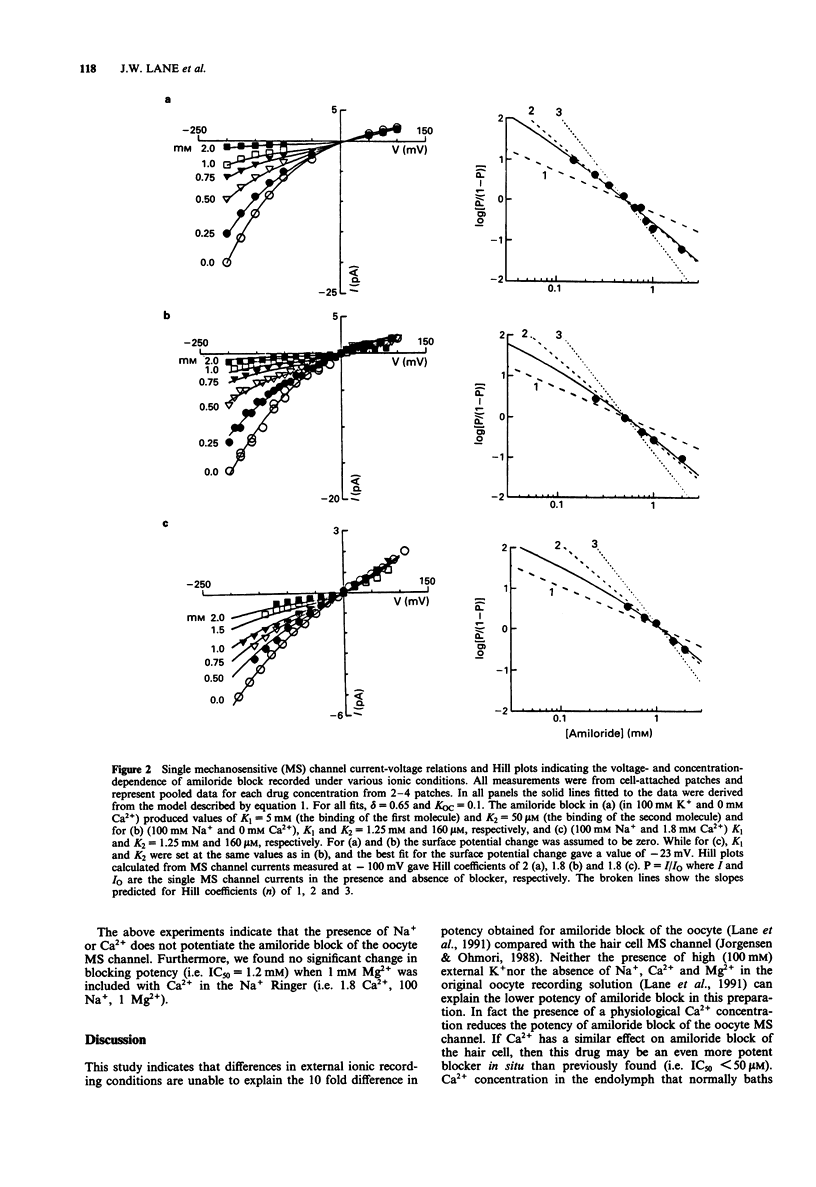

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benos D. J., Mandel L. J., Balaban R. S. On the mechanism of the amiloride-sodium entry site interaction in anuran skin epithelia. J Gen Physiol. 1979 Mar;73(3):307–326. doi: 10.1085/jgp.73.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Evans M. G., Fettiplace R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J Physiol. 1991 Mar;434:369–398. doi: 10.1113/jphysiol.1991.sp018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Wong P. Y. The role of calcium ions in the interaction of amiloride with membrane receptors. Mol Pharmacol. 1972 Mar;8(2):222–229. [PubMed] [Google Scholar]
- Desmedt L., Simaels J., Van Driessche W. Amiloride blockage of Na+ channels in amphibian epithelia does not require external Ca2+. Pflugers Arch. 1991 Dec;419(6):632–638. doi: 10.1007/BF00370307. [DOI] [PubMed] [Google Scholar]
- Garty H., Benos D. J. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988 Apr;68(2):309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Lane J. W., McBride D. W., Jr Amiloride: a molecular probe for mechanosensitive channels. Trends Pharmacol Sci. 1992 Oct;13(10):373–376. doi: 10.1016/0165-6147(92)90115-m. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Howard J., Roberts W. M., Hudspeth A. J. Mechanoelectrical transduction by hair cells. Annu Rev Biophys Biophys Chem. 1988;17:99–124. doi: 10.1146/annurev.bb.17.060188.000531. [DOI] [PubMed] [Google Scholar]
- Jørgensen F., Ohmori H. Amiloride blocks the mechano-electrical transduction channel of hair cells of the chick. J Physiol. 1988 Sep;403:577–588. doi: 10.1113/jphysiol.1988.sp017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. W., McBride D. W., Jr, Hamill O. P. Amiloride block of the mechanosensitive cation channel in Xenopus oocytes. J Physiol. 1991 Sep;441:347–366. doi: 10.1113/jphysiol.1991.sp018755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. W., McBride D. W., Jr, Hamill O. P. Structure-activity relations of amiloride and its analogues in blocking the mechanosensitive channel in Xenopus oocytes. Br J Pharmacol. 1992 Jun;106(2):283–286. doi: 10.1111/j.1476-5381.1992.tb14329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Taglietti V., Toselli M. A study of stretch-activated channels in the membrane of frog oocytes: interactions with Ca2+ ions. J Physiol. 1988 Dec;407:311–328. doi: 10.1113/jphysiol.1988.sp017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]


