Abstract
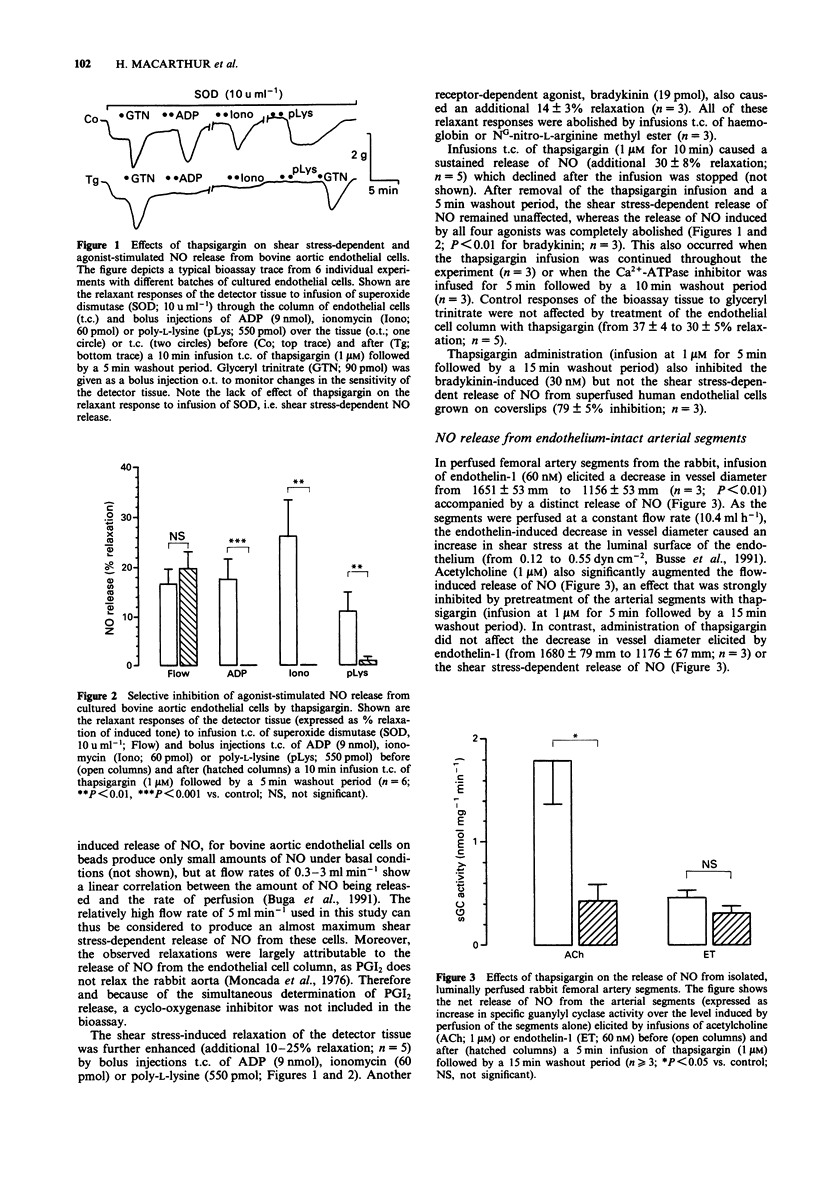
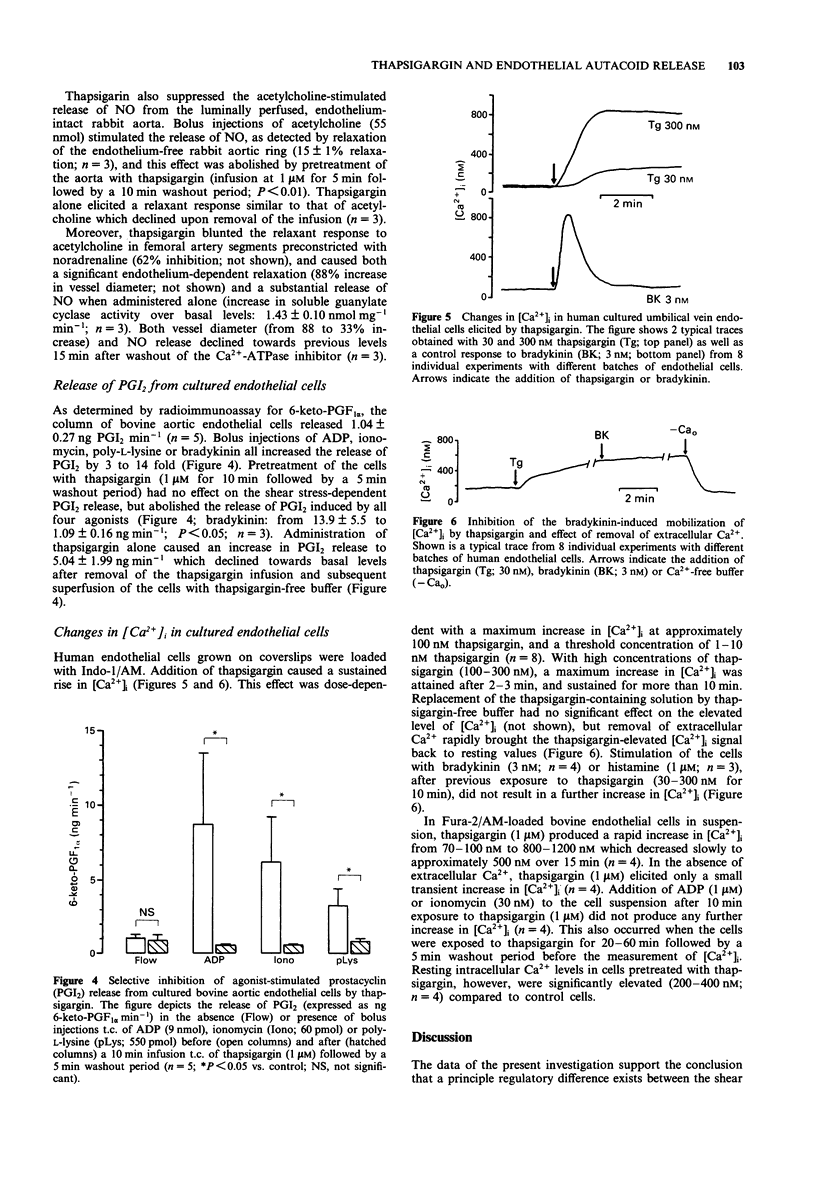
1. The effects of the Ca(2+)-ATPase inhibitor, thapsigargin, on the shear stress-dependent and on the agonist-stimulated release of endothelium-derived relaxing factor, i.e. nitric oxide (NO), and prostacyclin (PGI2) were studied in bovine and human cultured endothelial cells as well as in endothelium-intact arterial segments of the rabbit. 2. Preincubation with thapsigargin (1 microM for 10 min) had no effect on the shear stress-dependent release of NO from bovine aortic endothelial cells grown on beads, but abolished the release of NO induced by ADP, bradykinin, ionomycin or poly-L-lysine. Similarly, thapsigargin completely abrogated the agonist-stimulated PGI2 release from these cells, but had no effect on the shear stress-dependent release of PGI2. 3. The acetylcholine-induced release of NO from the luminally perfused thoracic aorta and femoral artery of the rabbit was suppressed by pretreatment with thapsigargin (1 microM). In contrast, thapsigargin did not affect the shear stress-dependent release of NO from the femoral artery. 4. Administration of thapsigargin to these vascular preparations or to cultured endothelial cells alone produced a substantial release of both NO and PGI2. This release declined towards previous values after washout of thapsigargin. 5. In human and bovine cultured endothelial cells, thapsigargin (1-1000 nM) caused a dose-dependent sustained rise in [Ca2+]i, an effect that was abolished in the absence of extracellular Ca2+. Stimulation of these cells with bradykinin, histamine, ADP or ionomycin after previous exposure to thapsigargin (30-1000 nM) no longer caused an increase in [Ca2+]i.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando J., Komatsuda T., Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev Biol. 1988 Sep;24(9):871–877. doi: 10.1007/BF02623896. [DOI] [PubMed] [Google Scholar]
- Buchan K. W., Martin W. Bradykinin induces elevations of cytosolic calcium through mobilisation of intracellular and extracellular pools in bovine aortic endothelial cells. Br J Pharmacol. 1991 Jan;102(1):35–40. doi: 10.1111/j.1476-5381.1991.tb12128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga G. M., Gold M. E., Fukuto J. M., Ignarro L. J. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991 Feb;17(2):187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- Busse R., Lamontagne D. Endothelium-derived bradykinin is responsible for the increase in calcium produced by angiotensin-converting enzyme inhibitors in human endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 1991 Jul;344(1):126–129. doi: 10.1007/BF00167392. [DOI] [PubMed] [Google Scholar]
- Dolor R. J., Hurwitz L. M., Mirza Z., Strauss H. C., Whorton A. R. Regulation of extracellular calcium entry in endothelial cells: role of intracellular calcium pool. Am J Physiol. 1992 Jan;262(1 Pt 1):C171–C181. doi: 10.1152/ajpcell.1992.262.1.C171. [DOI] [PubMed] [Google Scholar]
- Dull R. O., Davies P. F. Flow modulation of agonist (ATP)-response (Ca2+) coupling in vascular endothelial cells. Am J Physiol. 1991 Jul;261(1 Pt 2):H149–H154. doi: 10.1152/ajpheart.1991.261.1.H149. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Pollock J. S., Schmidt H. H., Heller M., Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R. V., Berk B. C., Alexander R. W., Nerem R. M. Flow-induced calcium transients in single endothelial cells: spatial and temporal analysis. Am J Physiol. 1992 Jun;262(6 Pt 1):C1411–C1417. doi: 10.1152/ajpcell.1992.262.6.C1411. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. EDRF coordinates the behaviour of vascular resistance vessels. Nature. 1987 Oct 1;329(6138):442–445. doi: 10.1038/329442a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Brüne B., Decker K., Ullrich V. The sulfhydryl reagent thimerosal elicits human platelet aggregation by mobilization of intracellular calcium and secondary prostaglandin endoperoxide formation. Biochem Biophys Res Commun. 1989 Mar 31;159(3):961–968. doi: 10.1016/0006-291x(89)92202-x. [DOI] [PubMed] [Google Scholar]
- Hecker M., Siegle I., Macarthur H., Sessa W. C., Vane J. R. Role of intracellular thiols in release of EDRF from cultured endothelial cells. Am J Physiol. 1992 Mar;262(3 Pt 2):H888–H896. doi: 10.1152/ajpheart.1992.262.3.H888. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Griffith T. M. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol. 1991 Jul;261(1 Pt 2):H257–H262. doi: 10.1152/ajpheart.1991.261.1.H257. [DOI] [PubMed] [Google Scholar]
- Jackson W. F., Mülsch A., Busse R. Rhythmic smooth muscle activity in hamster aortas is mediated by continuous release of NO from the endothelium. Am J Physiol. 1991 Jan;260(1 Pt 2):H248–H253. doi: 10.1152/ajpheart.1991.260.1.H248. [DOI] [PubMed] [Google Scholar]
- Koller A., Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol. 1990 Mar;258(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1990.258.3.H916. [DOI] [PubMed] [Google Scholar]
- Koller A., Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ Res. 1990 Aug;67(2):529–534. doi: 10.1161/01.res.67.2.529. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Refilling of endothelial calcium stores without bypassing the cytosol. FEBS Lett. 1990 Dec 10;276(1-2):108–110. doi: 10.1016/0014-5793(90)80519-o. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkumyants A. M., Balashov S. A., Veselova E. S., Khayutin V. M. Continuous control of the lumen of feline conduit arteries by blood flow rate. Cardiovasc Res. 1987 Dec;21(12):863–870. doi: 10.1093/cvr/21.12.863. [DOI] [PubMed] [Google Scholar]
- Mo M., Eskin S. G., Schilling W. P. Flow-induced changes in Ca2+ signaling of vascular endothelial cells: effect of shear stress and ATP. Am J Physiol. 1991 May;260(5 Pt 2):H1698–H1707. doi: 10.1152/ajpheart.1991.260.5.H1698. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Newby A. C., Henderson A. H. Stimulus-secretion coupling in vascular endothelial cells. Annu Rev Physiol. 1990;52:661–674. doi: 10.1146/annurev.ph.52.030190.003305. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pohl U., Holtz J., Busse R., Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986 Jan;8(1):37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Shen J., Luscinskas F. W., Connolly A., Dewey C. F., Jr, Gimbrone M. A., Jr Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am J Physiol. 1992 Feb;262(2 Pt 1):C384–C390. doi: 10.1152/ajpcell.1992.262.2.C384. [DOI] [PubMed] [Google Scholar]
- Smiesko V., Kozík J., Dolezel S. Role of endothelium in the control of arterial diameter by blood flow. Blood Vessels. 1985;22(5):247–251. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Hecker M., Ramwell P. W. Vascular activity of polycations and basic amino acids: L-arginine does not specifically elicit endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Jan 16;158(1):177–180. doi: 10.1016/s0006-291x(89)80194-9. [DOI] [PubMed] [Google Scholar]
- White D. G., Martin W. Differential control and calcium-dependence of production of endothelium-derived relaxing factor and prostacyclin by pig aortic endothelial cells. Br J Pharmacol. 1989 Jul;97(3):683–690. doi: 10.1111/j.1476-5381.1989.tb12004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]


