Abstract
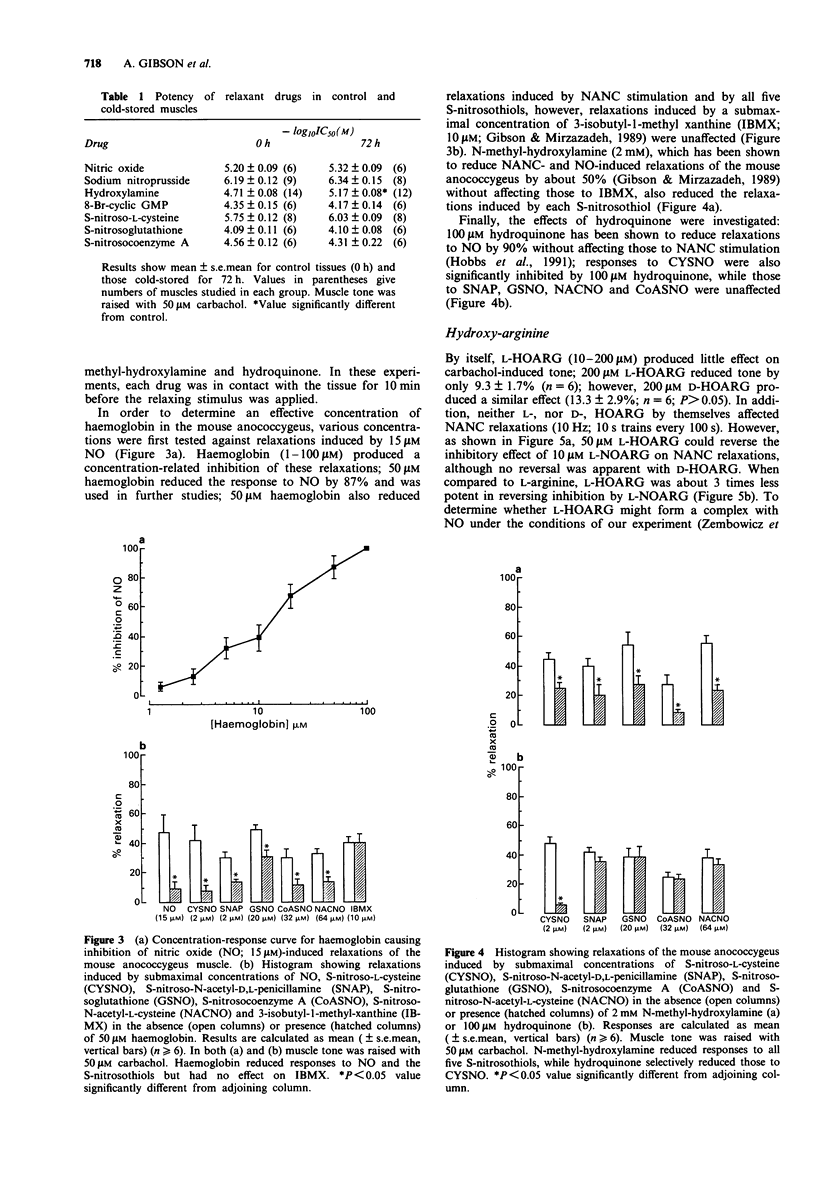
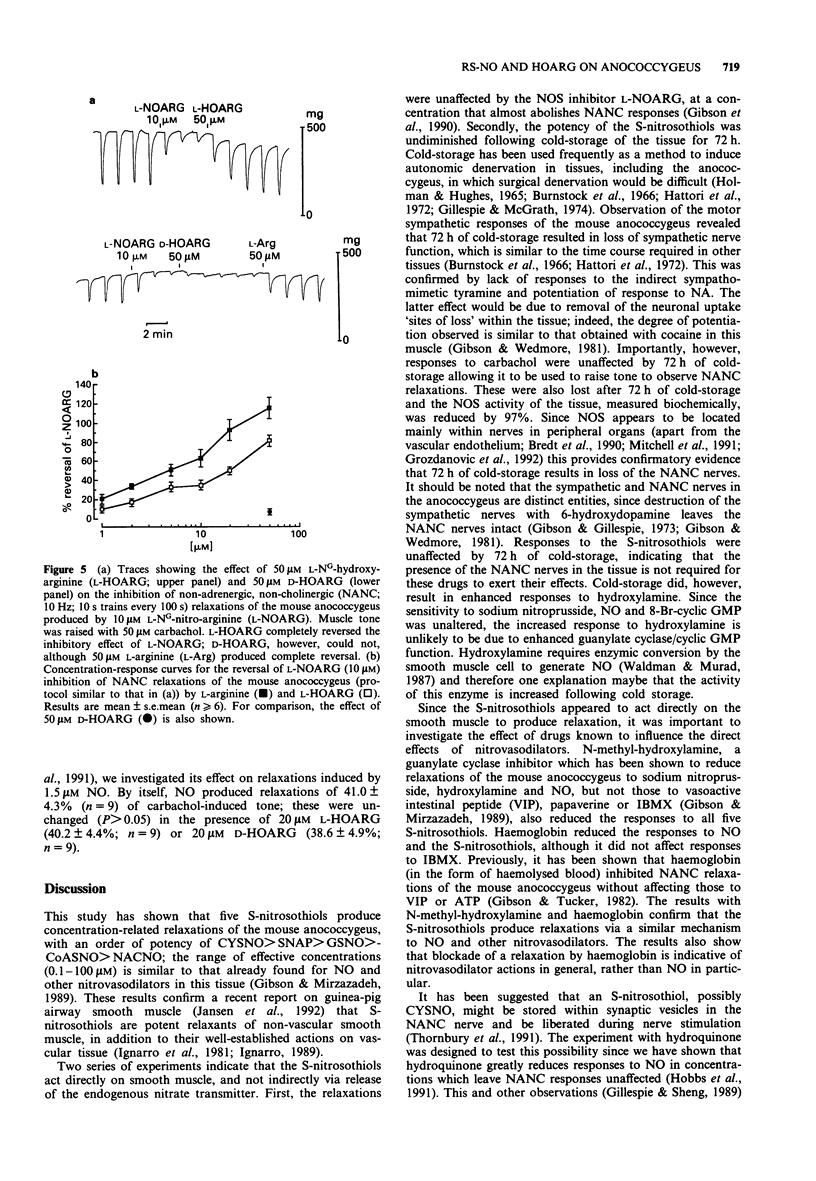
1. The effect of five S-nitrosothiols, and of the stereoisomers of NG-hydroxy-arginine (HOARG), were investigated on the mouse anococcygeus. 2. All five S-nitrosothiols produced concentration-related (0.1-100 microM) relaxations of carbachol (50 microM)-induced tone; the order of potency was S-nitroso-L-cysteine (CYSNO) > S-nitroso-N-acetyl-D,L-penicillamine (SNAP) > S-nitrosoglutathione (GSNO) > S-nitrosocoenzyme A (CoASNO) > S-nitroso-N-acetyl-L-cysteine (NACNO). The relaxations were unaffected by the nitric oxide synthase (NOS) inhibitor, L-NG-nitro-arginine (10 microM) (L-NOARG). 3. Cold-storage of the tissue for 72 h resulted in loss of sympathetic and non-adrenergic, non-cholinergic (NANC) nerve function. NOS activity in the tissue was reduced by 97%. Despite this, relaxations induced by the S-nitrosothiols were unaffected. 4. Haemoglobin (50 microM) attenuated relaxations induced by NO and the S-nitrosothiols, although responses to 3-isobutyl-1-methyl-xanthine were unaffected. N-methyl-hydroxylamine (2 mM) which has been shown previously to produce selective inhibition of NANC and nitrovasodilator responses in this tissue, also reduced responses to all S-nitrosothiols. 5. Hydroquinone (100 microM) greatly reduced relaxations to CYSNO (by 88%) but had no effect on those to SNAP, GSNO, CoASNO or NACNO. Since hydroquinone does not reduce responses to NANC stimulation, CYSNO is unlikely to be the NANC transmitter. 6. L-HOARG by itself (up to 100 microM) had no significant effect on carbachol-induced tone or on NANC (10 Hz; 10 strain every 100 s) relaxations.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Rand M. J. The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol. 1966 Feb;182(3):504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer M. A., Bredt D. S., Snyder S. H. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991 May 15;176(3):1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- Gibson A., Gillespie J. S. The effect of immunosympathectomy and of 6-hydroxydopamine on the responses of the rat anococcygeus to nerve stimulation and to some drugs. Br J Pharmacol. 1973 Feb;47(2):261–267. doi: 10.1111/j.1476-5381.1973.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs. Br J Pharmacol. 1989 Mar;96(3):637–644. doi: 10.1111/j.1476-5381.1989.tb11863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Tucker J. F. The effects of vasoactive intestinal polypeptide and of adenosine 5'-triphosphate on the isolated anococcygeus muscle of the mouse. Br J Pharmacol. 1982 Sep;77(1):97–103. doi: 10.1111/j.1476-5381.1982.tb09274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Wedmore C. V. Responses of the isolated anococcygeus muscle of the mouse to drugs and to field stimulation. J Auton Pharmacol. 1981 Jun;1(3):225–233. doi: 10.1111/j.1474-8673.1981.tb00451.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., McGrath J. C. The response of the cat anococcygeus muscle to nerve or drug stimulation and a comparison with the rat anococcygeus. Br J Pharmacol. 1974 Jan;50(1):109–118. doi: 10.1111/j.1476-5381.1974.tb09597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. A comparison of haemoglobin and erythrocytes as inhibitors of smooth muscle relaxation by the NANC transmitter in the BRP and rat anococcygeus and by EDRF in the rabbit aortic strip. Br J Pharmacol. 1989 Oct;98(2):445–450. doi: 10.1111/j.1476-5381.1989.tb12616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanovic Z., Baumgarten H. G., Brüning G. Histochemistry of NADPH-diaphorase, a marker for neuronal nitric oxide synthase, in the peripheral autonomic nervous system of the mouse. Neuroscience. 1992;48(1):225–235. doi: 10.1016/0306-4522(92)90351-2. [DOI] [PubMed] [Google Scholar]
- HOLMAN M. E., HUGHES J. R. INHIBITION OF INTESTINAL SMOOTH MUSCLE. Aust J Exp Biol Med Sci. 1965 Jun;43:277–290. doi: 10.1038/icb.1965.27. [DOI] [PubMed] [Google Scholar]
- Hattori K., Kurahashi K., Mori J., Shibata S. The effect of cold storage on the adrenergic mechanisms of intestinal smooth muscle. Br J Pharmacol. 1972 Nov;46(3):423–437. doi: 10.1111/j.1476-5381.1972.tb08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Tucker J. F., Gibson A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br J Pharmacol. 1991 Nov;104(3):645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Jansen A., Drazen J., Osborne J. A., Brown R., Loscalzo J., Stamler J. S. The relaxant properties in guinea pig airways of S-nitrosothiols. J Pharmacol Exp Ther. 1992 Apr;261(1):154–160. [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- McCall T., Vallance P. Nitric oxide takes centre-stage with newly defined roles. Trends Pharmacol Sci. 1992 Jan;13(1):1–6. doi: 10.1016/0165-6147(92)90002-n. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Sheng H., Förstermann U., Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Ramagopal M. V., Leighton H. J. Effects of NG-monomethyl-L-arginine on field stimulation-induced decreases in cytosolic Ca2+ levels and relaxation in the rat anococcygeus muscle. Eur J Pharmacol. 1989 Dec 19;174(2-3):297–299. doi: 10.1016/0014-2999(89)90325-7. [DOI] [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F., Griffith O. W., Feldman P. L., Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991 Apr 5;266(10):6259–6263. [PubMed] [Google Scholar]
- Thornbury K. D., Ward S. M., Dalziel H. H., Carl A., Westfall D. P., Sanders K. M. Nitric oxide and nitrosocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am J Physiol. 1991 Sep;261(3 Pt 1):G553–G557. doi: 10.1152/ajpgi.1991.261.3.G553. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Wallace G. C., Fukuto J. M. Synthesis and bioactivity of N omega-hydroxyarginine: a possible intermediate in the biosynthesis of nitric oxide from arginine. J Med Chem. 1991 May;34(5):1746–1748. doi: 10.1021/jm00109a032. [DOI] [PubMed] [Google Scholar]
- Zembowicz A., Hecker M., Macarthur H., Sessa W. C., Vane J. R. Nitric oxide and another potent vasodilator are formed from NG-hydroxy-L-arginine by cultured endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11172–11176. doi: 10.1073/pnas.88.24.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]


