Abstract
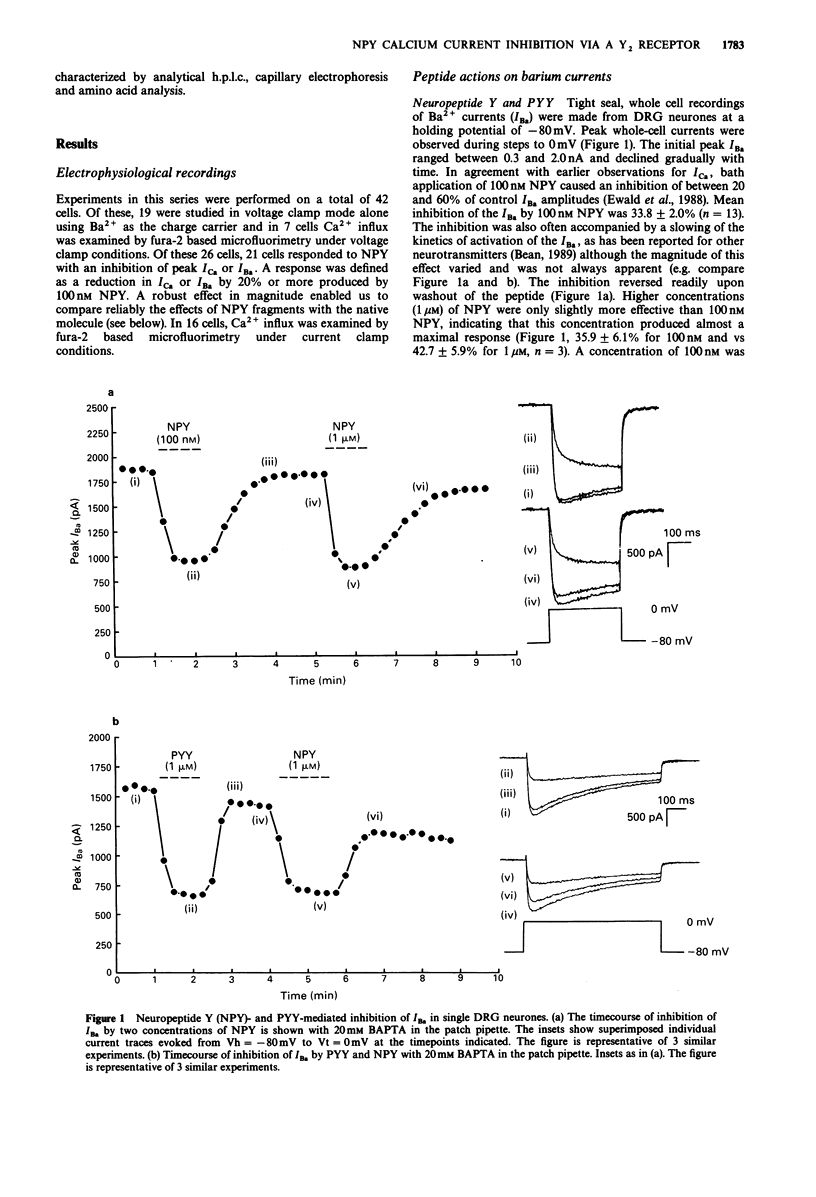
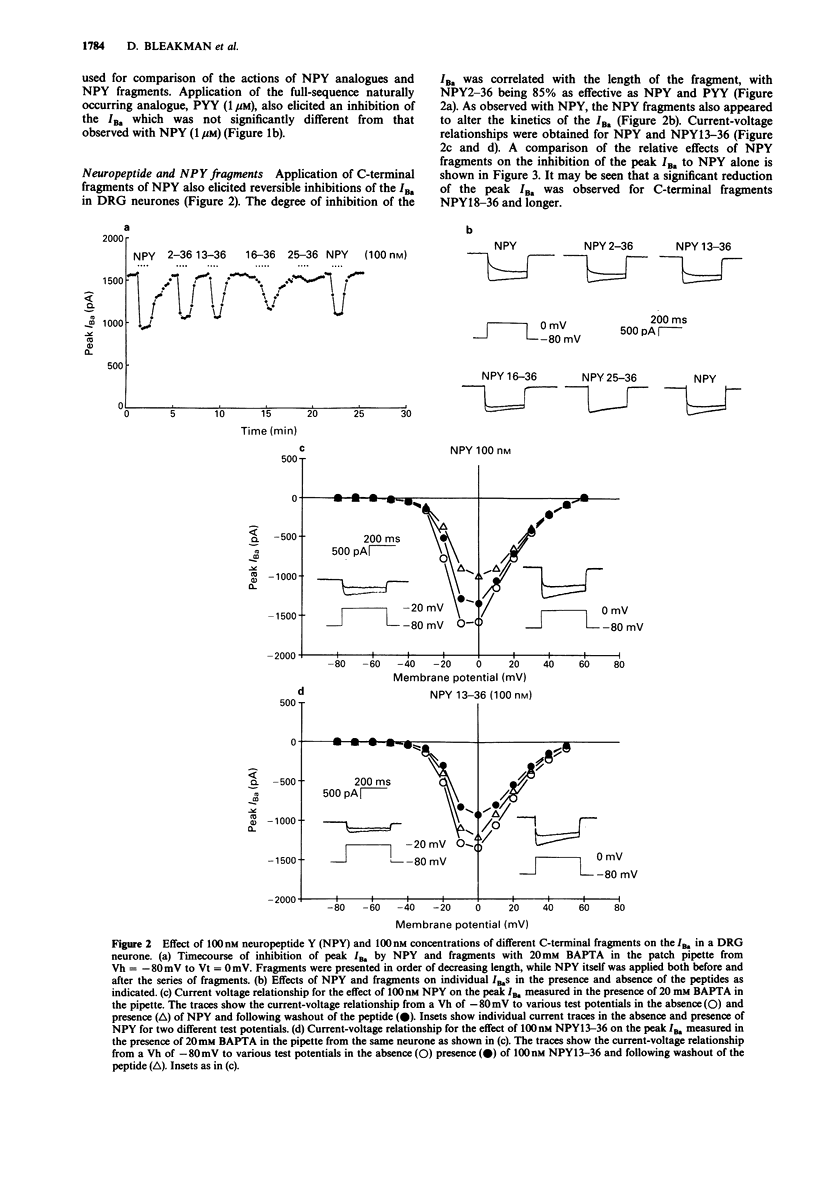
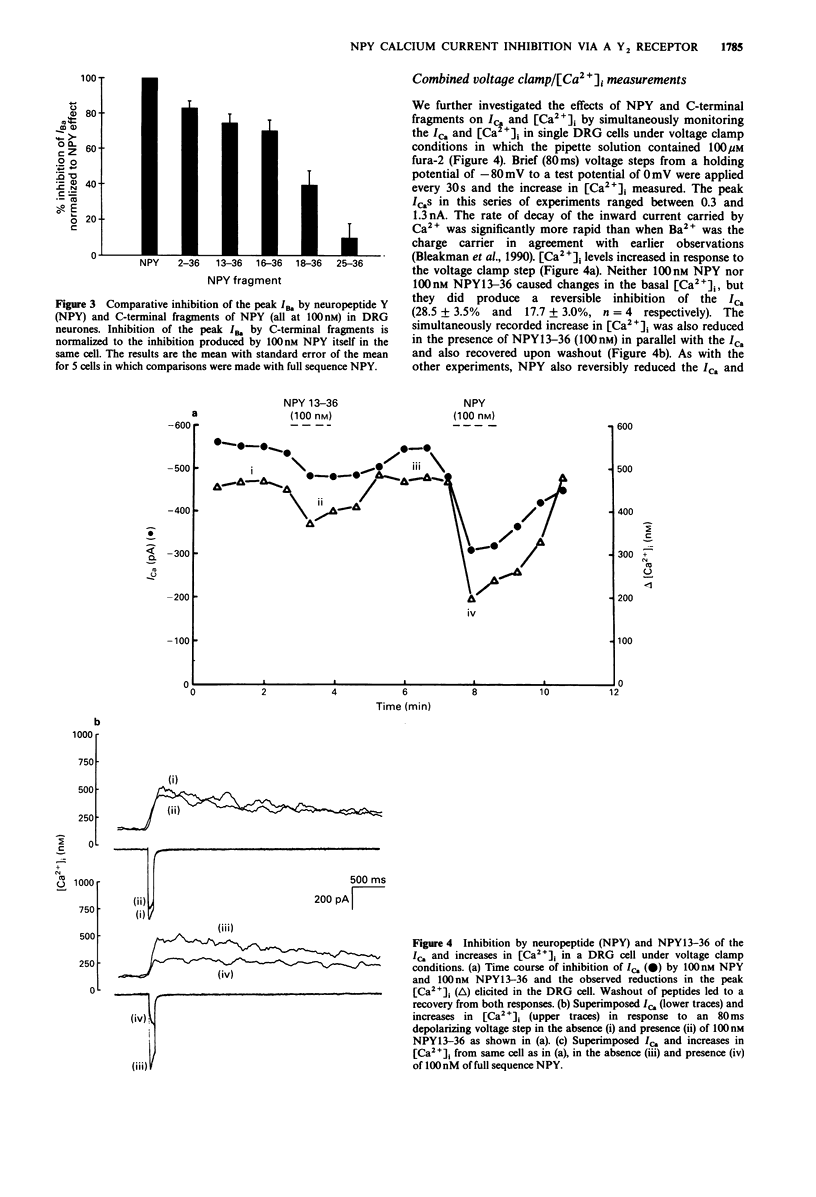
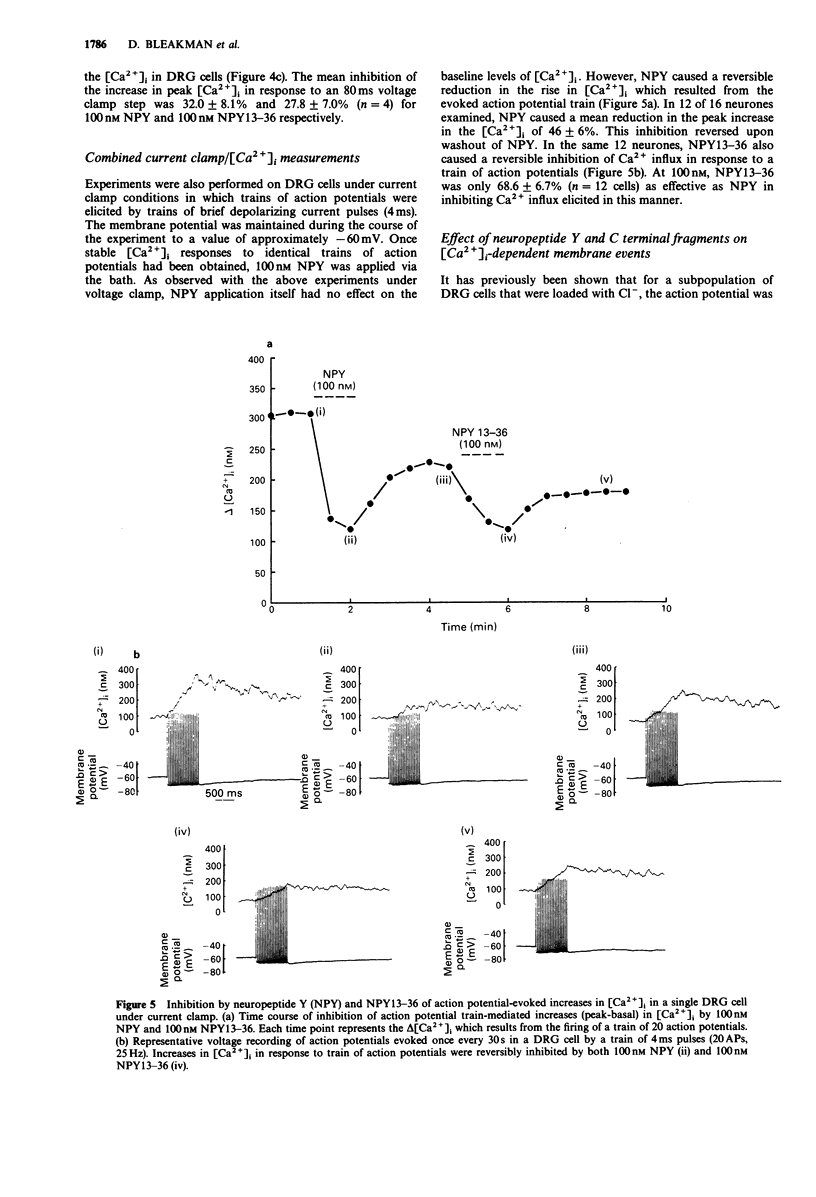
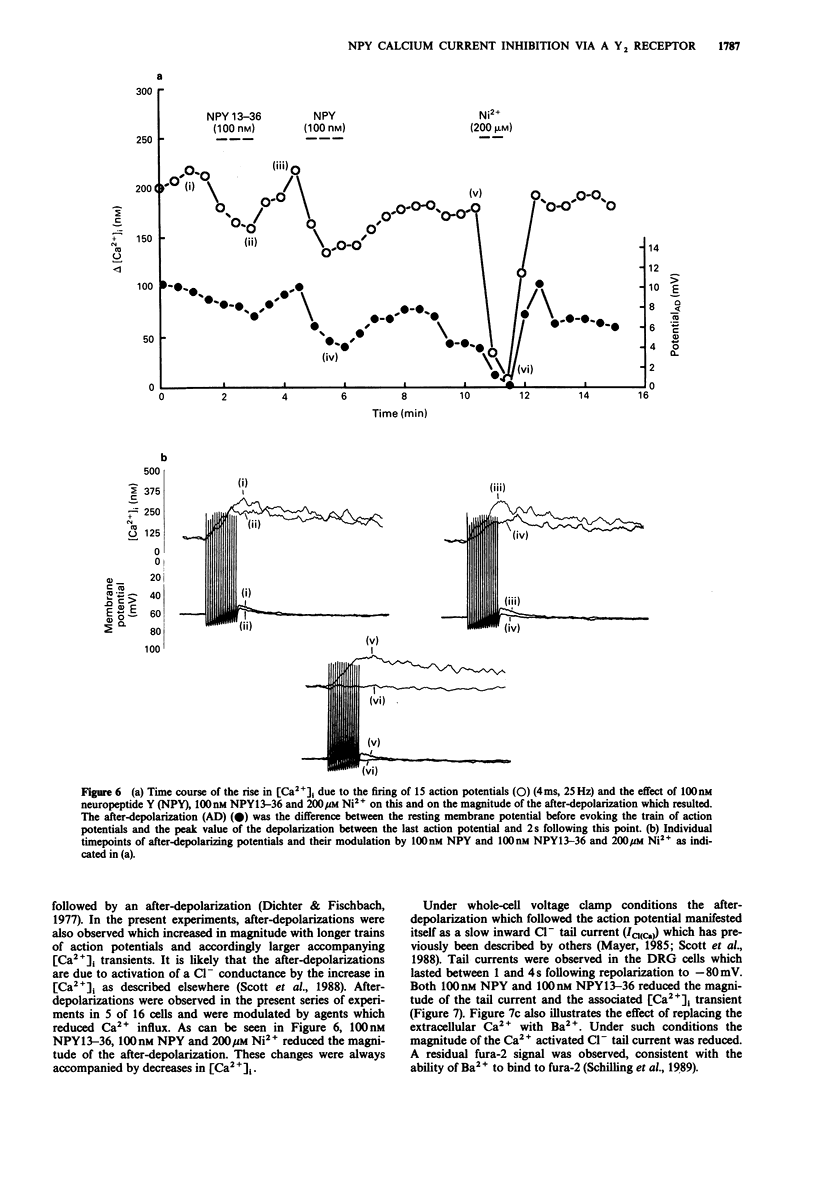
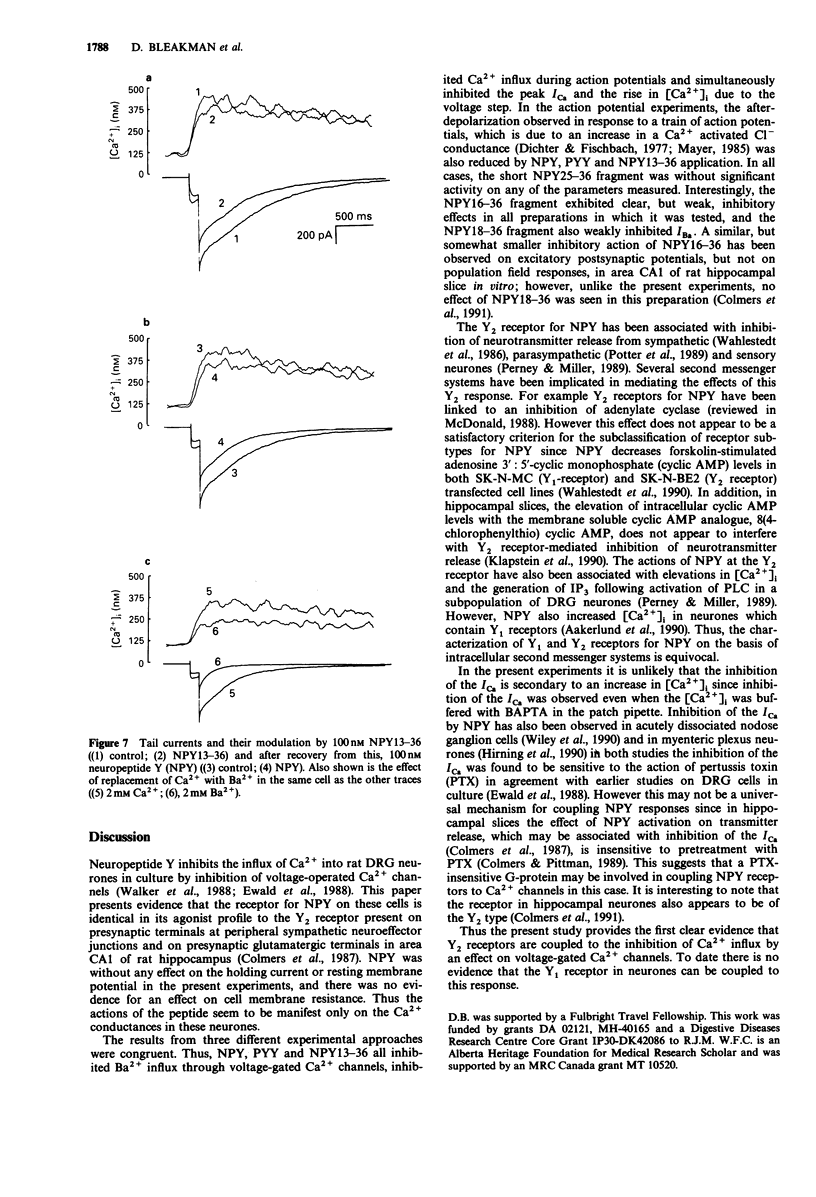
1. The identity of the neuropeptide Y (NPY) receptor associated with the observed inhibition of neuronal Ca2+ currents (ICa) in rat dorsal root ganglion (DRG) cells has been established on the basis of agonist responses to analogues and carboxy terminal (C-terminal) fragments of the NPY molecule. 2. Whole cell barium currents (IBa) in DRG cells were reversibly inhibited by 100 nM NPY, 100 nM PYY and C-terminal fragments of NPY in a manner that correlated with the length of the NPY fragments (for inhibition of the IBa NPY = PYY greater than NPY2-36 greater than NPY13-36 greater than NPY16-36 greater than NPY18-36 much greater than NPY25-36). 3. C-terminal fragments of NPY were also effective in reversibly reducing the ICa, the associated increase in the intracellular Ca2+ concentration [( Ca2+]i) and the increased [Ca2+]i produced by evoked action potentials in the DRG cells. In addition, a Ca(2+)-activated Cl- conductance was also reversibly reduced by NPY fragments only when accompanied by a reduction in Ca2+ entry. 4. We conclude that the Y2 receptor for neuropeptide Y is coupled to inhibition of Ca2+ influx via voltage-sensitive calcium channels in DRG cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aakerlund L., Gether U., Fuhlendorff J., Schwartz T. W., Thastrup O. Y1 receptors for neuropeptide Y are coupled to mobilization of intracellular calcium and inhibition of adenylate cyclase. FEBS Lett. 1990 Jan 15;260(1):73–78. doi: 10.1016/0014-5793(90)80069-u. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Bleakman D., Brorson J. R., Miller R. J. The effect of capsaicin on voltage-gated calcium currents and calcium signals in cultured dorsal root ganglion cells. Br J Pharmacol. 1990 Oct;101(2):423–431. doi: 10.1111/j.1476-5381.1990.tb12725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers W. F., Klapstein G. J., Fournier A., St-Pierre S., Treherne K. A. Presynaptic inhibition by neuropeptide Y in rat hippocampal slice in vitro is mediated by a Y2 receptor. Br J Pharmacol. 1991 Jan;102(1):41–44. doi: 10.1111/j.1476-5381.1991.tb12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers W. F., Lukowiak K., Pittman Q. J. Neuropeptide Y action in the rat hippocampal slice: site and mechanism of presynaptic inhibition. J Neurosci. 1988 Oct;8(10):3827–3837. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers W. F., Lukowiak K., Pittman Q. J. Presynaptic action of neuropeptide Y in area CA1 of the rat hippocampal slice. J Physiol. 1987 Feb;383:285–299. doi: 10.1113/jphysiol.1987.sp016409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers W. F., Pittman Q. J. Presynaptic inhibition by neuropeptide Y and baclofen in hippocampus: insensitivity to pertussis toxin treatment. Brain Res. 1989 Sep 25;498(1):99–104. doi: 10.1016/0006-8993(89)90403-4. [DOI] [PubMed] [Google Scholar]
- Dichter M. A., Fischbach G. D. The action potential of chick dorsal root ganglion neurones maintained in cell culture. J Physiol. 1977 May;267(2):281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald D. A., Matthies H. J., Perney T. M., Walker M. W., Miller R. J. The effect of down regulation of protein kinase C on the inhibitory modulation of dorsal root ganglion neuron Ca2+ currents by neuropeptide Y. J Neurosci. 1988 Jul;8(7):2447–2451. doi: 10.1523/JNEUROSCI.08-07-02447.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald D. A., Pang I. H., Sternweis P. C., Miller R. J. Differential G protein-mediated coupling of neurotransmitter receptors to Ca2+ channels in rat dorsal root ganglion neurons in vitro. Neuron. 1989 Feb;2(2):1185–1193. doi: 10.1016/0896-6273(89)90185-2. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Forest M., Fournier A. BOP reagent for the coupling of pGlu and Boc-His(Tos) in solid phase peptide synthesis. Int J Pept Protein Res. 1990 Feb;35(2):89–94. doi: 10.1111/j.1399-3011.1990.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Fournier A., Wang C. T., Felix A. M. Applications of BOP reagent in solid phase synthesis. Advantages of BOP reagent for difficult couplings exemplified by a synthesis of [Ala 15]-GRF(1-29)-NH2. Int J Pept Protein Res. 1988 Jan;31(1):86–97. doi: 10.1111/j.1399-3011.1988.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., Miller R. J. Inhibition of calcium currents in cultured myenteric neurons by neuropeptide Y: evidence for direct receptor/channel coupling. Brain Res. 1990 Nov 5;532(1-2):120–130. doi: 10.1016/0006-8993(90)91751-2. [DOI] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. K. NPY and related substances. Crit Rev Neurobiol. 1988;4(1):97–135. [PubMed] [Google Scholar]
- Perney T. M., Miller R. J. Two different G-proteins mediate neuropeptide Y and bradykinin-stimulated phospholipid breakdown in cultured rat sensory neurons. J Biol Chem. 1989 May 5;264(13):7317–7327. [PubMed] [Google Scholar]
- Pietta P. G., Cavallo P. F., Takahashi K., Marshall G. R. Preparation and use of benzhydrylamine polymers in peptide synthesis. II. Syntheses of thyrotropin releasing hormone, thyrocalcitonin 26-32, and eledoisin. J Org Chem. 1974 Jan 11;39(1):44–48. doi: 10.1021/jo00915a008. [DOI] [PubMed] [Google Scholar]
- Potter E. K., Mitchell L., McCloskey M. J., Tseng A., Goodman A. E., Shine J., McCloskey D. I. Pre- and postjunctional actions of neuropeptide Y and related peptides. Regul Pept. 1989 May;25(2):167–177. doi: 10.1016/0167-0115(89)90258-9. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Scott R. H., McGuirk S. M., Dolphin A. C. Modulation of divalent cation-activated chloride ion currents. Br J Pharmacol. 1988 Jul;94(3):653–662. doi: 10.1111/j.1476-5381.1988.tb11572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Sturek M., Miller R. J. Measurement of neuronal Ca2+ transients using simultaneous microfluorimetry and electrophysiology. Pflugers Arch. 1988 Jul;412(1-2):216–223. doi: 10.1007/BF00583753. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Grundemar L., Håkanson R., Heilig M., Shen G. H., Zukowska-Grojec Z., Reis D. J. Neuropeptide Y receptor subtypes, Y1 and Y2. Ann N Y Acad Sci. 1990;611:7–26. doi: 10.1111/j.1749-6632.1990.tb48918.x. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Yanaihara N., Håkanson R. Evidence for different pre-and post-junctional receptors for neuropeptide Y and related peptides. Regul Pept. 1986 Feb;13(3-4):307–318. doi: 10.1016/0167-0115(86)90048-0. [DOI] [PubMed] [Google Scholar]
- Walker M. W., Ewald D. A., Perney T. M., Miller R. J. Neuropeptide Y modulates neurotransmitter release and Ca2+ currents in rat sensory neurons. J Neurosci. 1988 Jul;8(7):2438–2446. doi: 10.1523/JNEUROSCI.08-07-02438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. W., Gross R. A., Lu Y. X., Macdonald R. L. Neuropeptide Y reduces calcium current and inhibits acetylcholine release in nodose neurons via a pertussis toxin-sensitive mechanism. J Neurophysiol. 1990 Jun;63(6):1499–1507. doi: 10.1152/jn.1990.63.6.1499. [DOI] [PubMed] [Google Scholar]


