Abstract
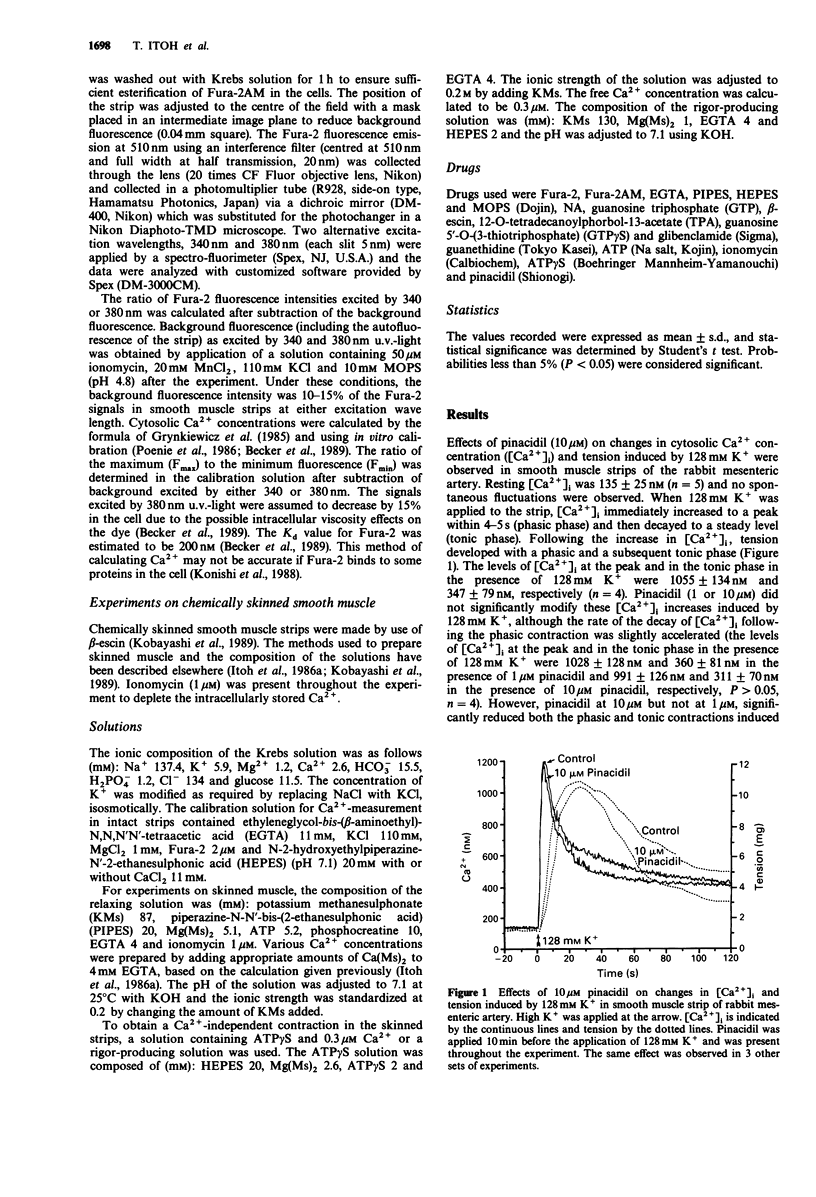
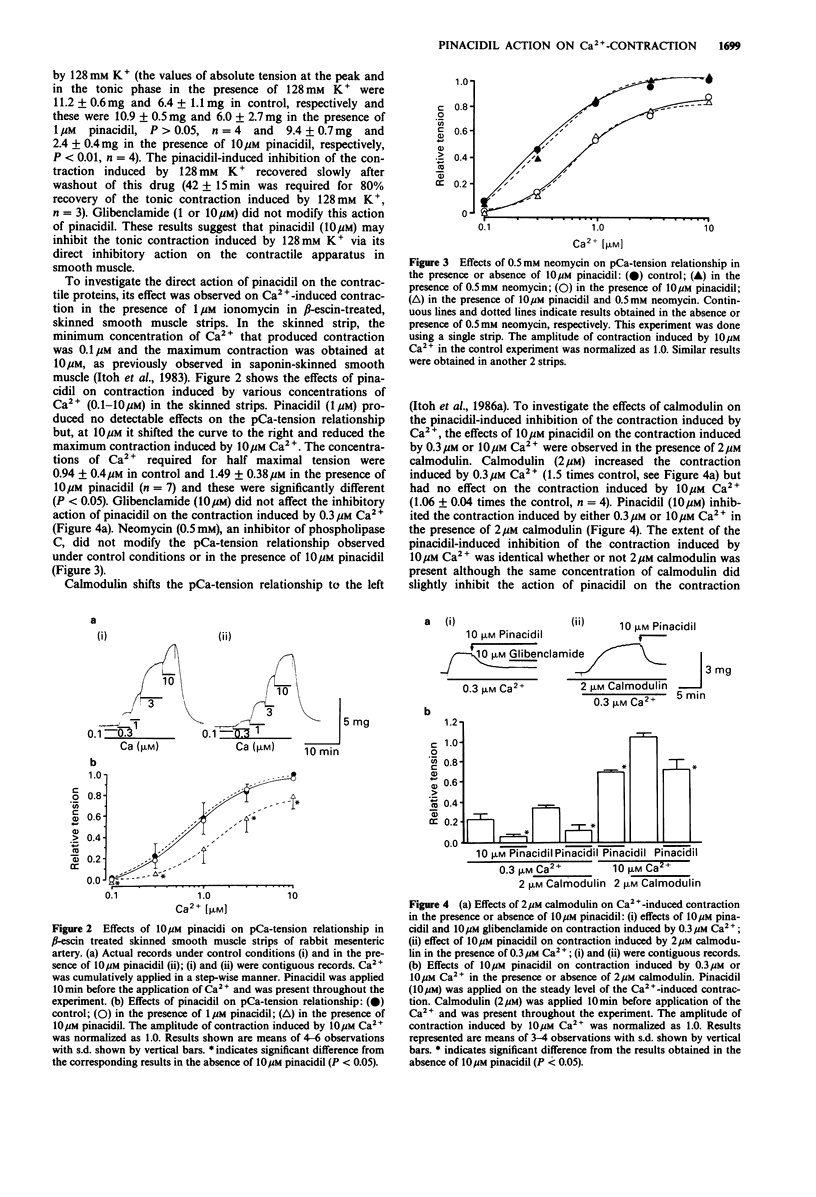
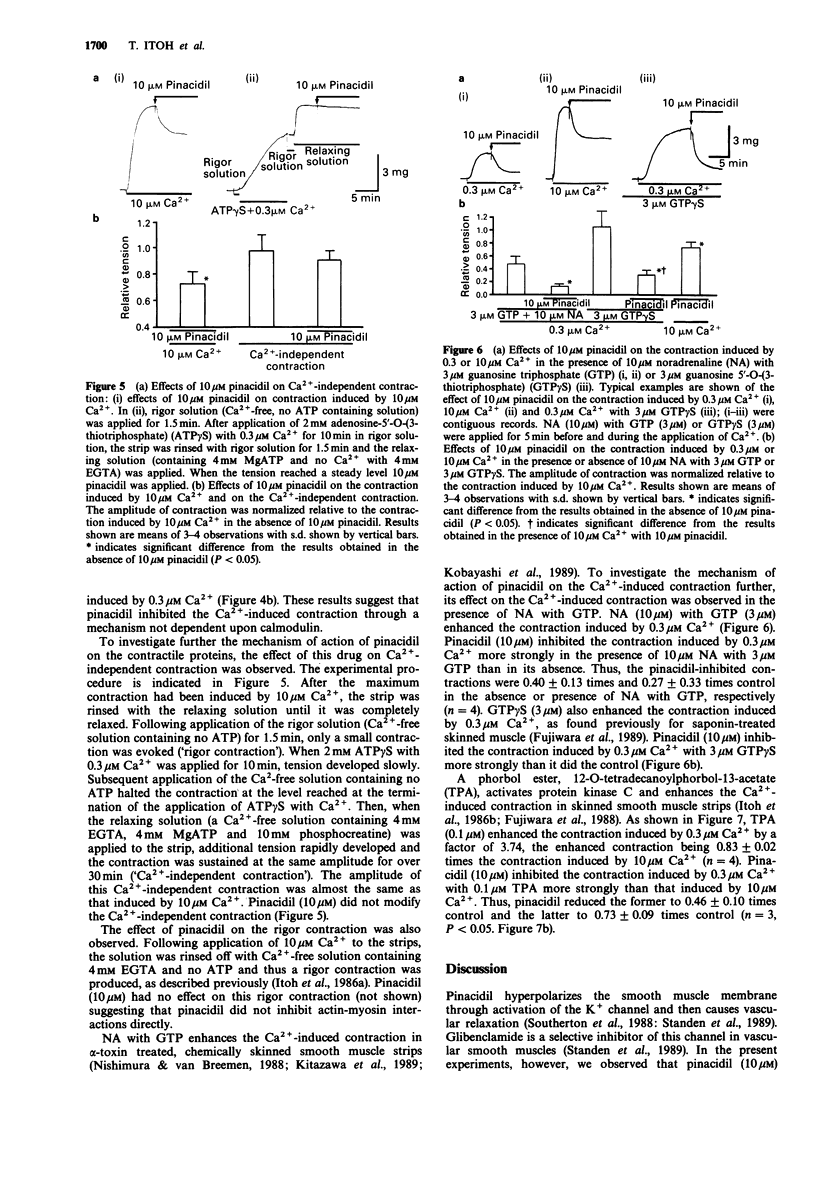
1. The effects of pinacidil were investigated on changes in cellular Ca2+ concentration ([Ca2+]i) and tension in intact and chemically skinned smooth muscle strips of the rabbit mesenteric artery. 2. High K+ (128 mM) produced a large phasic followed by a tonic increase in [Ca2+]i and tension in intact muscle strips. Pinacidil at 10 microM but not 1 microM, inhibited the phasic and tonic contractions induced by 128 mM K+ without a corresponding change in [Ca2+]i. 3. In beta-escin-treated skinned smooth muscle, the minimum Ca2+ concentration that produced contraction was 0.1 microM and the maximum contraction was obtained at 10 microM. Pinacidil at 10 microM but not 1 microM, shifted the pCa-tension relation curve to the right and also inhibited the maximum contraction induced by Ca2+. The concentrations of Ca2+ required for half maximal tension were 0.9 microM in control and 1.5 microM in the presence of 10 microM pinacidil. Calmodulin (2 microM) increased the contraction induced by 0.3 microM Ca2+ (but not by 10 microM Ca2+) in the skinned strips. Pinacidil (10 microM) inhibited the contraction induced by 0.3 microM or 10 microM Ca2+ in the presence of 2 microM calmodulin. 4. Noradrenaline (NA, 10 microM) with guanosine triphosphate (GTP, 3 microM), guanosine 5'-O-(3-thiotriphosphate) (GTP gamma S, 3 microM) or 12-O-tetradecanoylphorbol-13-acetate (TPA, 0.1 microM) all enhanced the contraction induced by 0.3 microM Ca2+. Pinacidil (10 microM) inhibited the contraction induced by 0.3 microM Ca2+ more strongly in the presence of the above agents than in their absence.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. G-proteins, the inositol lipid signalling pathway, and secretion. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):247–265. doi: 10.1098/rstb.1988.0075. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Itoh T., Kubota Y., Kuriyama H. Actions of a phorbol ester on factors regulating contraction in rabbit mesenteric artery. Circ Res. 1988 Nov;63(5):893–902. doi: 10.1161/01.res.63.5.893. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Itoh T., Kubota Y., Kuriyama H. Effects of guanosine nucleotides on skinned smooth muscle tissue of the rabbit mesenteric artery. J Physiol. 1989 Jan;408:535–547. doi: 10.1113/jphysiol.1989.sp017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J Physiol. 1986 Jul;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H., Sumimoto K. A phorbol ester has dual actions on the mechanical response in the rabbit mesenteric and porcine coronary arteries. J Physiol. 1986 Jun;375:515–534. doi: 10.1113/jphysiol.1986.sp016131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M., Li Y., Seki N., Kuriyama H. Pinacidil inhibits neuromuscular transmission indirectly in the guinea-pig and rabbit mesenteric arteries. Br J Pharmacol. 1990 Nov;101(3):581–586. doi: 10.1111/j.1476-5381.1990.tb14124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Quast U., Cook N. S. Moving together: K+ channel openers and ATP-sensitive K+ channels. Trends Pharmacol Sci. 1989 Nov;10(11):431–435. doi: 10.1016/S0165-6147(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Satoh S., Kubota Y., Itoh T., Kuriyama H. Mechanisms of the Ba2+-induced contraction in smooth muscle cells of the rabbit mesenteric artery. J Gen Physiol. 1987 Feb;89(2):215–237. doi: 10.1085/jgp.89.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southerton J. S., Weston A. H., Bray K. M., Newgreen D. T., Taylor S. G. The potassium channel opening action of pinacidil; studies using biochemical, ion flux and microelectrode techniques. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):310–318. doi: 10.1007/BF00173406. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Videbaek L. M., Aalkjaer C., Mulvany M. J. Pinacidil opens K+-selective channels causing hyperpolarization and relaxation of noradrenaline contractions in rat mesenteric resistance vessels. Br J Pharmacol. 1988 Sep;95(1):103–108. doi: 10.1111/j.1476-5381.1988.tb16553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



