Abstract
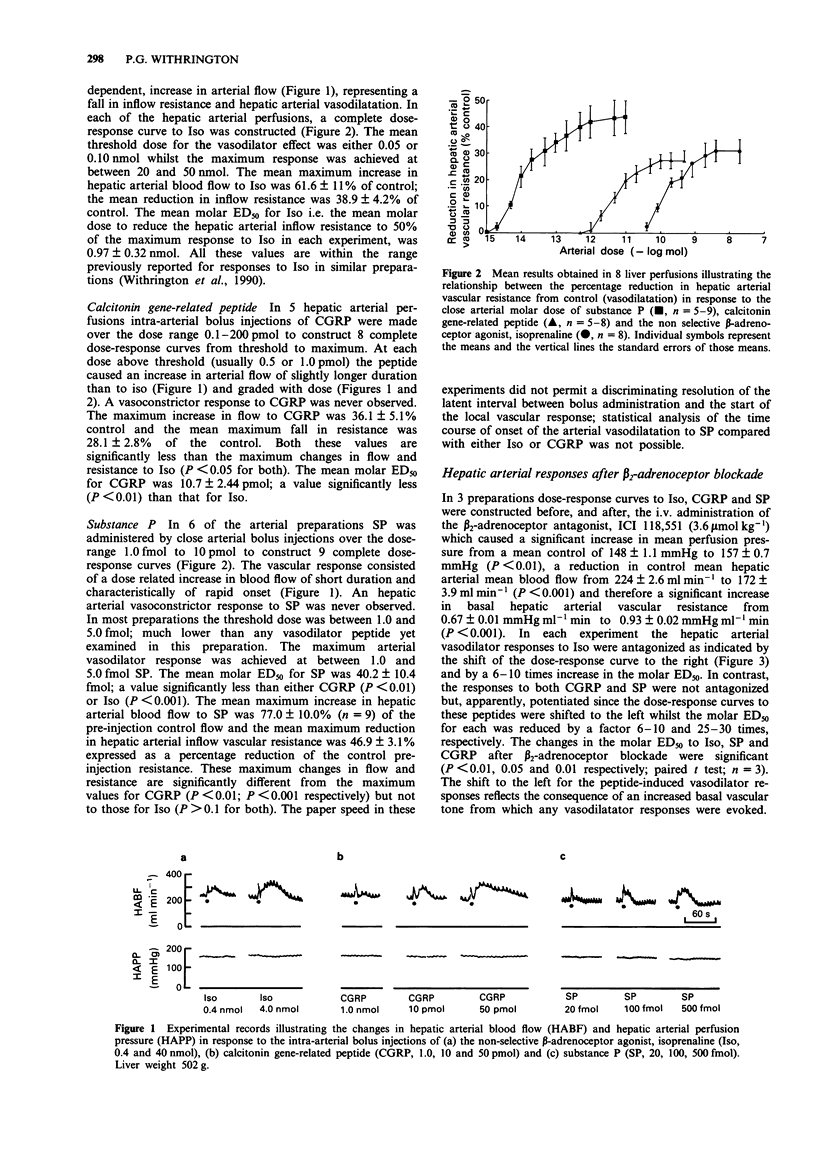
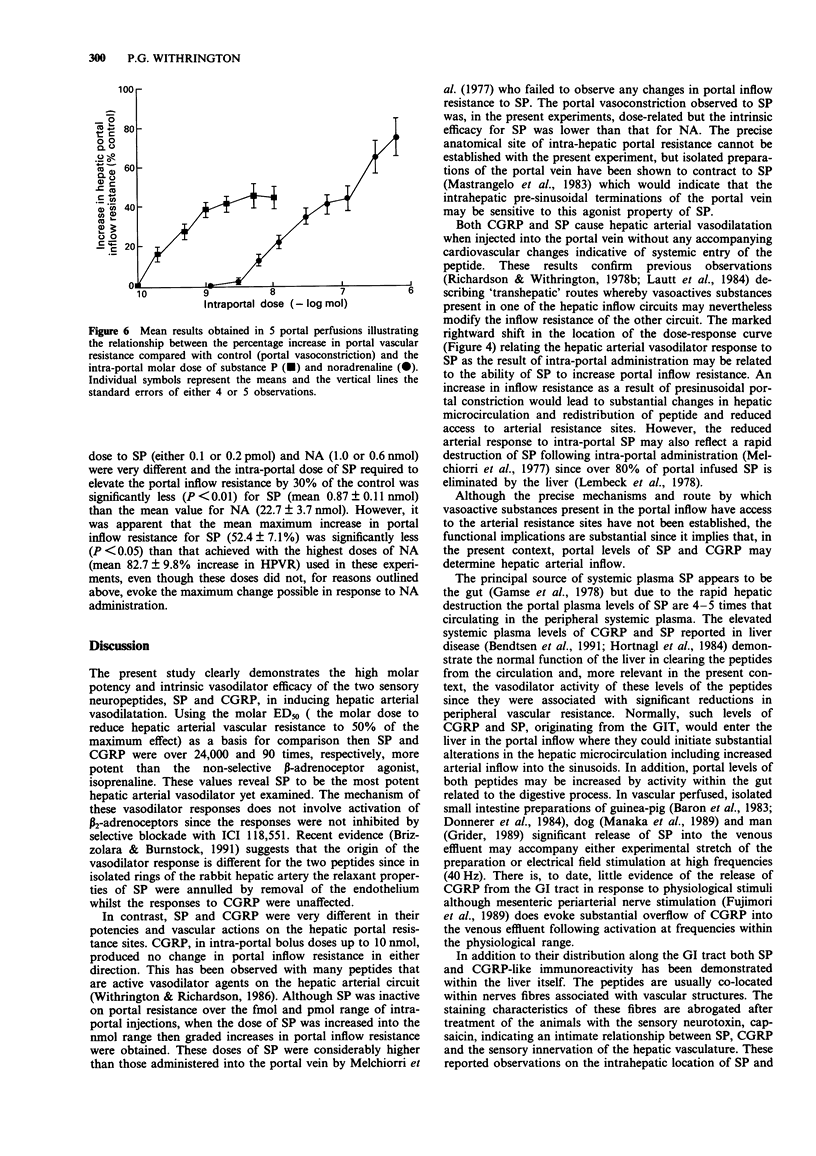
1. The two peptides, calcitonin gene-related peptide (CGRP) and substance P (SP) were administered individually as bolus injections into the separately perfused hepatic arterial and portal vascular beds of the anaesthetized dog to assess their actions and relative molar potencies at these sites. 2. CGRP caused an immediate dose-related increase in hepatic arterial flow when injected close-arterially, reflecting a fall in resistance. This vasodilator effect was slightly increased by the prior administration of the selective beta 2-adrenoceptor antagonist, ICI 118,551. 3. On a molar basis, CGRP was more potent as an hepatic arterial vasodilator than the non-selective beta-adrenoceptor agonist, isoprenaline (Iso). 4. Intra-portal injection of CGRP also evoked hepatic arterial vasodilatation unaccompanied by other cardiovascular changes. 5. CGRP in doses up to 10 nmol had no effect on portal vascular resistance when administered intra-portally. 6. SP evoked a rapid, dose-related increase in hepatic arterial flow when injected intra-arterially. The molar ED50 for this hepatic vasodilatation was 40.2 fmol, significantly less than the ED50 for either CGRP or Iso. SP was the most potent hepatic arterial vasodilator yet examined. The vasodilator effect of SP was slightly potentiated by prior beta 2-adrenoceptor blockade. 7. SP caused hepatic arterial vasodilatation when administered by intra-portal injection; its absolute and relative potency was much reduced. 8. SP when injected intra-portally caused a graded increase in hepatic portal inflow resistance. The molar potency for this portal vasoconstriction was significantly greater than that for noradrenaline (NA); however, the maximum increase in portal resistance was significantly less to SP than to NA.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Niijima A., Jacobs H. L. An hepatic osmoreceptor mechanism in the rat: electrophysiological and behavioral studies. Am J Physiol. 1976 Oct;231(4):1043–1049. doi: 10.1152/ajplegacy.1976.231.4.1043. [DOI] [PubMed] [Google Scholar]
- Adachi A., Niijima A. Thermosensitive afferent fibers in the hepatic branch of the vagus nerve in the guinea pig. J Auton Nerv Syst. 1982 Mar;5(2):101–109. doi: 10.1016/0165-1838(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Andrews W. H., Orbach J. Sodium receptors activating some nerves of perfused rabbit livers. Am J Physiol. 1974 Dec;227(6):1273–1275. doi: 10.1152/ajplegacy.1974.227.6.1273. [DOI] [PubMed] [Google Scholar]
- Barja F., Mathison R. Sensory innervation of the rat portal vein and the hepatic artery. J Auton Nerv Syst. 1984 Apr;10(2):117–125. doi: 10.1016/0165-1838(84)90050-x. [DOI] [PubMed] [Google Scholar]
- Baron S. A., Jaffe B. M., Gintzler A. R. Release of substance P from the enteric nervous system: direct quantitation and characterization. J Pharmacol Exp Ther. 1983 Nov;227(2):365–368. [PubMed] [Google Scholar]
- Bendtsen F., Schifter S., Henriksen J. H. Increased circulating calcitonin gene-related peptide (CGRP) in cirrhosis. J Hepatol. 1991 Jan;12(1):118–123. doi: 10.1016/0168-8278(91)90920-7. [DOI] [PubMed] [Google Scholar]
- Brizzolara A. L., Burnstock G. Endothelium-dependent and endothelium-independent vasodilatation of the hepatic artery of the rabbit. Br J Pharmacol. 1991 May;103(1):1206–1212. doi: 10.1111/j.1476-5381.1991.tb12325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlei F., Lygidakis N. J., Speranza V., Brummelkamp W. H., McGurrin J. F., Pietroletti R., Lezoche E., Bostwick D. G. Neuroendocrine innervation of the hepatic vessels in the rat and in man. J Surg Res. 1988 Nov;45(5):417–426. doi: 10.1016/0022-4804(88)90191-6. [DOI] [PubMed] [Google Scholar]
- Corder R., Withrington P. G. The actions of neuropeptide Y and peptide YY on the hepatic arterial and portal vascular beds of the anaesthetized dog. Br J Pharmacol. 1988 Aug;94(4):1149–1156. doi: 10.1111/j.1476-5381.1988.tb11633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnerer J., Barthó L., Holzer P., Lembeck F. Intestinal peristalsis associated with release of immunoreactive substance P. Neuroscience. 1984 Apr;11(4):913–918. doi: 10.1016/0306-4522(84)90202-1. [DOI] [PubMed] [Google Scholar]
- Fujimori A., Saito A., Kimura S., Watanabe T., Uchiyama Y., Kawasaki H., Goto K. Neurogenic vasodilation and release of calcitonin gene-related peptide (CGRP) from perivascular nerves in the rat mesenteric artery. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1391–1398. doi: 10.1016/0006-291x(89)92758-7. [DOI] [PubMed] [Google Scholar]
- Gamse R., Mroz E., Leeman S., Lembeck F. The intestine as source of immunoreactive substance P in plasma of the cat. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;305(1):17–21. doi: 10.1007/BF00497001. [DOI] [PubMed] [Google Scholar]
- Goehler L. E., Sternini C., Brecha N. C. Calcitonin gene-related peptide immunoreactivity in the biliary pathway and liver of the guinea-pig: distribution and colocalization with substance P. Cell Tissue Res. 1988 Jul;253(1):145–150. doi: 10.1007/BF00221749. [DOI] [PubMed] [Google Scholar]
- Grider J. R. Identification of neurotransmitters regulating intestinal peristaltic reflex in humans. Gastroenterology. 1989 Dec;97(6):1414–1419. doi: 10.1016/0016-5085(89)90384-3. [DOI] [PubMed] [Google Scholar]
- Hörtnagl H., Singer E. A., Lenz K., Kleinberger G., Lochs H. Substance P is markedly increased in plasma of patients with hepatic coma. Lancet. 1984 Mar 3;1(8375):480–483. doi: 10.1016/s0140-6736(84)92851-4. [DOI] [PubMed] [Google Scholar]
- Lautt W. W., Legare D. J., d'Almeida M. S. Adenosine as putative regulator of hepatic arterial flow (the buffer response). Am J Physiol. 1985 Mar;248(3 Pt 2):H331–H338. doi: 10.1152/ajpheart.1985.248.3.H331. [DOI] [PubMed] [Google Scholar]
- Lautt W. W., MacLachlan T. L., Brown L. C. The effect of hypertonic infusions on hepatic blood flows and liver volume in the cat. Can J Physiol Pharmacol. 1977 Dec;55(6):1339–1344. doi: 10.1139/y77-179. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P., Schweditsch M., Gamse R. Elimination of substance P from the circulation of the rat and its inhibition by bacitracin. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;305(1):9–16. doi: 10.1007/BF00497000. [DOI] [PubMed] [Google Scholar]
- Manaka H., Manaka Y., Kostolanska F., Fox J. E., Daniel E. E. Release of VIP and substance P from isolated perfused canine ileum. Am J Physiol. 1989 Aug;257(2 Pt 1):G182–G190. doi: 10.1152/ajpgi.1989.257.2.G182. [DOI] [PubMed] [Google Scholar]
- Marshall I., Al-Kazwini S. J., Holman J. J., Craig R. K. Human and rat alpha-CGRP but not calcitonin cause mesenteric vasodilatation in rats. Eur J Pharmacol. 1986 Apr 16;123(2):217–222. doi: 10.1016/0014-2999(86)90662-x. [DOI] [PubMed] [Google Scholar]
- Mastrangelo D., Mathison R., Huggel H. Postjunctional localization of substance P receptors on the rat portal vein. Pharmacology. 1983;27(6):305–318. doi: 10.1159/000137885. [DOI] [PubMed] [Google Scholar]
- Niijima A. Glucose-sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea-pig. J Physiol. 1982 Nov;332:315–323. doi: 10.1113/jphysiol.1982.sp014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijima A. Afferent discharges from venous pressoreceptors in liver. Am J Physiol. 1977 Jan;232(1):C76–C81. doi: 10.1152/ajpcell.1977.232.1.C76. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Richardson P. D., Withrington P. G. Alpha and beta adrenoceptors in the hepatic portal venous vascular bed of the dog [proceedings]. Br J Pharmacol. 1978 Mar;62(3):376P–377P. [PMC free article] [PubMed] [Google Scholar]
- Richardson P. D., Withrington P. G. Pressure-flow relationships and effects of noradrenaline and isoprenaline on the hepatic arterial and portal venous vascular beds of the dog. J Physiol. 1978 Sep;282:451–470. doi: 10.1113/jphysiol.1978.sp012475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Kamada T., Hayashi N., Sato N., Kasahara A., Fusamoto H., Shiosaka S., Tohyama M., Shiotani Y. Immunohistochemical distribution of glucagon, substance P and vasoactive intestinal polypeptide in hepatic vasculature of the rat. Hepatology. 1984 Nov-Dec;4(6):1184–1189. doi: 10.1002/hep.1840040614. [DOI] [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Ekblad E., Håkanson R., Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. 1986 Aug;15(1):1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Withrington P. G., Dhume V. G., Croxton R., Gerbes A. L. The actions of human atrial natriuretic factor on hepatic arterial and portal vascular beds of the anaesthetized dog. Br J Pharmacol. 1990 Apr;99(4):810–814. doi: 10.1111/j.1476-5381.1990.tb13011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrington P. G. The relaxant properties of human calcitonin gene-related peptide on vascular and extravascular capsular) smooth muscle of the isolated blood-perfused spleen of the anaesthetized dog. Br J Pharmacol. 1989 Apr;96(4):823–828. doi: 10.1111/j.1476-5381.1989.tb11890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


