Abstract
1. The effect of NG-nitro-L-arginine methyl ester (L-NAME) and NG-nitro-L-arginine (L-NOARG) on noradrenaline (NA)-induced contractility and acetylcholine (ACh)-induced endothelium-dependent relaxation was studied in rat mesenteric resistance arteries. 2. Third order branches of mesenteric arteries were dissected and mounted on two forty micron wires in a Mulvany myograph. 3. Incubation with L-NAME and L-NOARG (10 microM) caused a time-dependent shift in the 50% response to NA (ED50) (0.01 microM-10 microM) but was not associated with an increase in the maximum contractile response. 4. L-NAME and L-NOARG (10 microM) caused a time-dependent inhibition of ACh (1 microM)-induced relaxation with a maximum effect after 120 min. 5. Following endothelium removal, incubation with either L-NAME or L-NOARG caused no significant shift in the ED50, although the residual relaxation response to ACh (1 microM) was further attenuated. 6. Incubation with the cyclo-oxygenase inhibitor, indomethacin, enhanced the relaxation to ACh and reduced the inhibitory effects of L-NAME and L-NOARG. 7. In conclusion, L-NAME and L-NOARG are potent inhibitors of acetylcholine-induced endothelium-dependent relaxation in mesenteric resistance arteries. The shift in ED50 associated with these inhibitors suggests a probable role for the endothelium in modulating the contractility of the resistance vasculature.
Full text
PDF

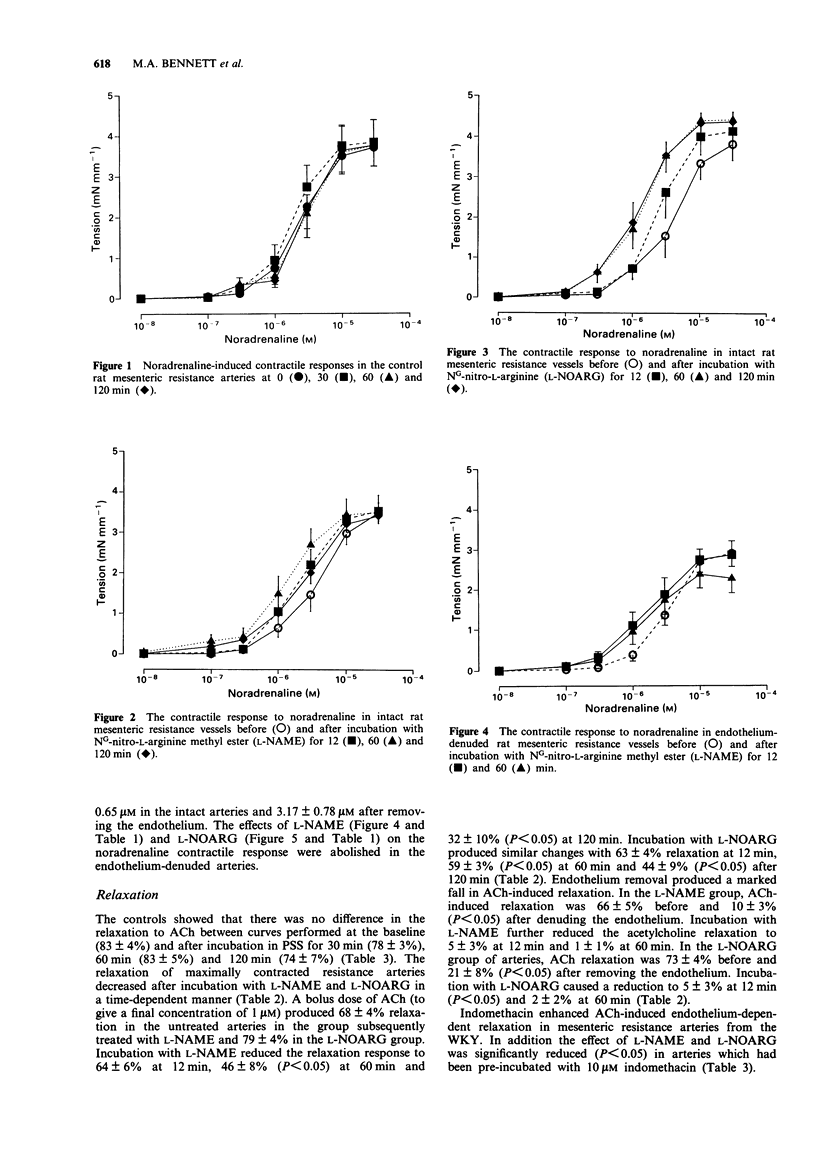
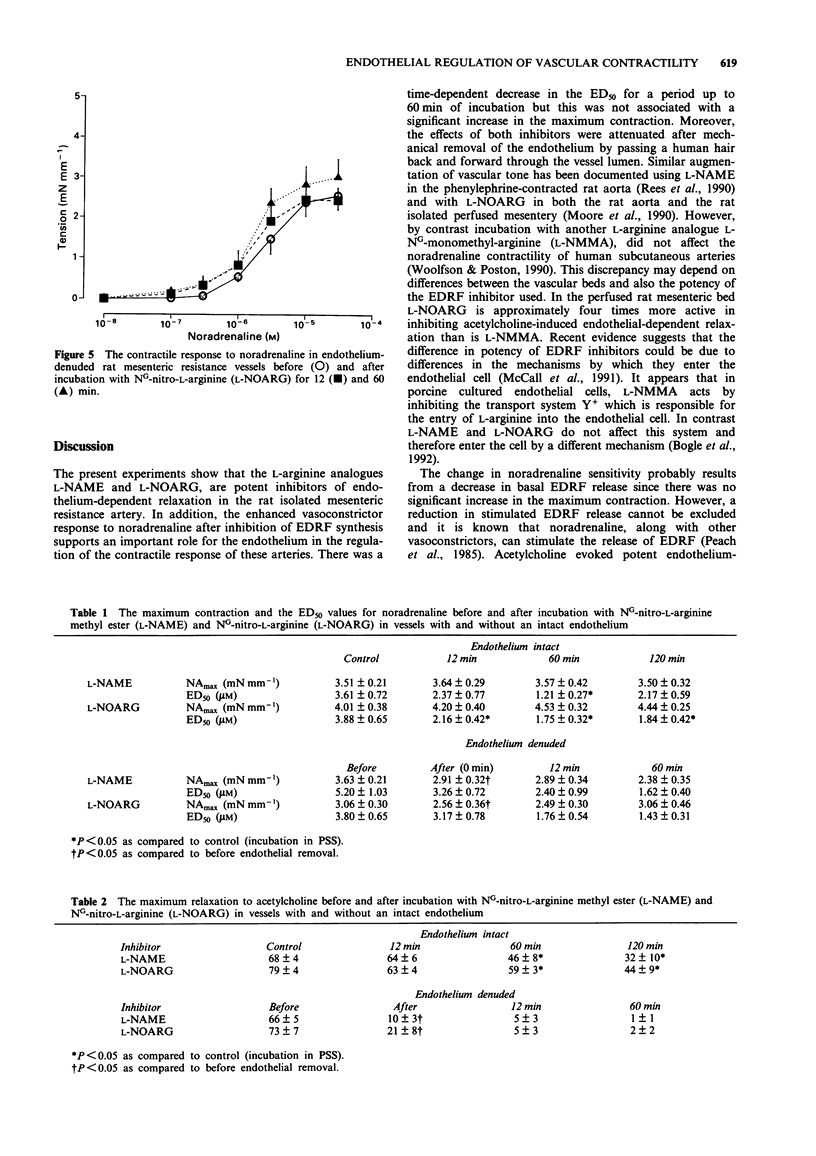


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Heagerty A. M., Swales J. D., Thurston H. Endothelial-dependent relaxation in human subcutaneous resistance vessels. Blood Vessels. 1987;24(1-2):85–88. doi: 10.1159/000158674. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Moncada S., Pearson J. D., Mann G. E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br J Pharmacol. 1992 Apr;105(4):768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Collins P., Lewis M. J., Henderson A. H. Endothelium derived relaxant factor. J R Coll Physicians Lond. 1985 Apr;19(2):74–79. [PMC free article] [PubMed] [Google Scholar]
- Harder D. R. Pressure-induced myogenic activation of cat cerebral arteries is dependent on intact endothelium. Circ Res. 1987 Jan;60(1):102–107. doi: 10.1161/01.res.60.1.102. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Aarhus L. L., Vanhoutte P. M. Indomethacin improves the impaired endothelium-dependent relaxations in small mesenteric arteries of the spontaneously hypertensive rat. Am J Hypertens. 1990 Jan;3(1):55–58. doi: 10.1093/ajh/3.1.55. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Feelisch M., Palmer R. M., Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991 Jan;102(1):234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Mechanical properties of vascular smooth muscle cells in situ. Nature. 1976 Apr 15;260(5552):617–619. doi: 10.1038/260617a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Osol G., Cipolla M., Knutson S. A new method for mechanically denuding the endothelium of small (50-150 microns) arteries with a human hair. Blood Vessels. 1989;26(5):320–324. doi: 10.1159/000158781. [DOI] [PubMed] [Google Scholar]
- Owen M. P., Bevan J. A. Acetylcholine induced endothelial-dependent vasodilation increases as artery diameter decreases in the rabbit ear. Experientia. 1985 Aug 15;41(8):1057–1058. doi: 10.1007/BF01952142. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Loeb A. L., Singer H. A., Saye J. Endothelium-derived vascular relaxing factor. Hypertension. 1985 May-Jun;7(3 Pt 2):I94–100. doi: 10.1161/01.hyp.7.3_pt_2.i94. [DOI] [PubMed] [Google Scholar]
- Raij L., Lüscher T. F., Vanhoutte P. M. High potassium diet augments endothelium-dependent relaxations in the Dahl rat. Hypertension. 1988 Dec;12(6):562–567. doi: 10.1161/01.hyp.12.6.562. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Nitric oxide synthesised from L-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc Res. 1989 Dec;23(12):1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- Van de Voorde J., Vanheel B., Leusen I. Endothelium-dependent relaxation and hyperpolarization in aorta from control and renal hypertensive rats. Circ Res. 1992 Jan;70(1):1–8. doi: 10.1161/01.res.70.1.1. [DOI] [PubMed] [Google Scholar]
- Watt P. A., Thurston H. Endothelium-dependent relaxation in resistance vessels from the spontaneously hypertensive rats. J Hypertens. 1989 Aug;7(8):661–666. doi: 10.1097/00004872-198908000-00010. [DOI] [PubMed] [Google Scholar]
- Wiest E., Trach V., Dämmgen J. Removal of endothelial function in coronary resistance vessels by saponin. Basic Res Cardiol. 1989 Sep-Oct;84(5):469–478. doi: 10.1007/BF01908199. [DOI] [PubMed] [Google Scholar]
- Woolfson R. G., Poston L. Effect of NG-monomethyl-L-arginine on endothelium-dependent relaxation of human subcutaneous resistance arteries. Clin Sci (Lond) 1990 Sep;79(3):273–278. doi: 10.1042/cs0790273. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., von Segesser L., Bauer E., Stulz P., Turina M., Lüscher T. F. Different activation of the endothelial L-arginine and cyclooxygenase pathway in the human internal mammary artery and saphenous vein. Circ Res. 1991 Jan;68(1):52–60. doi: 10.1161/01.res.68.1.52. [DOI] [PubMed] [Google Scholar]


