Abstract
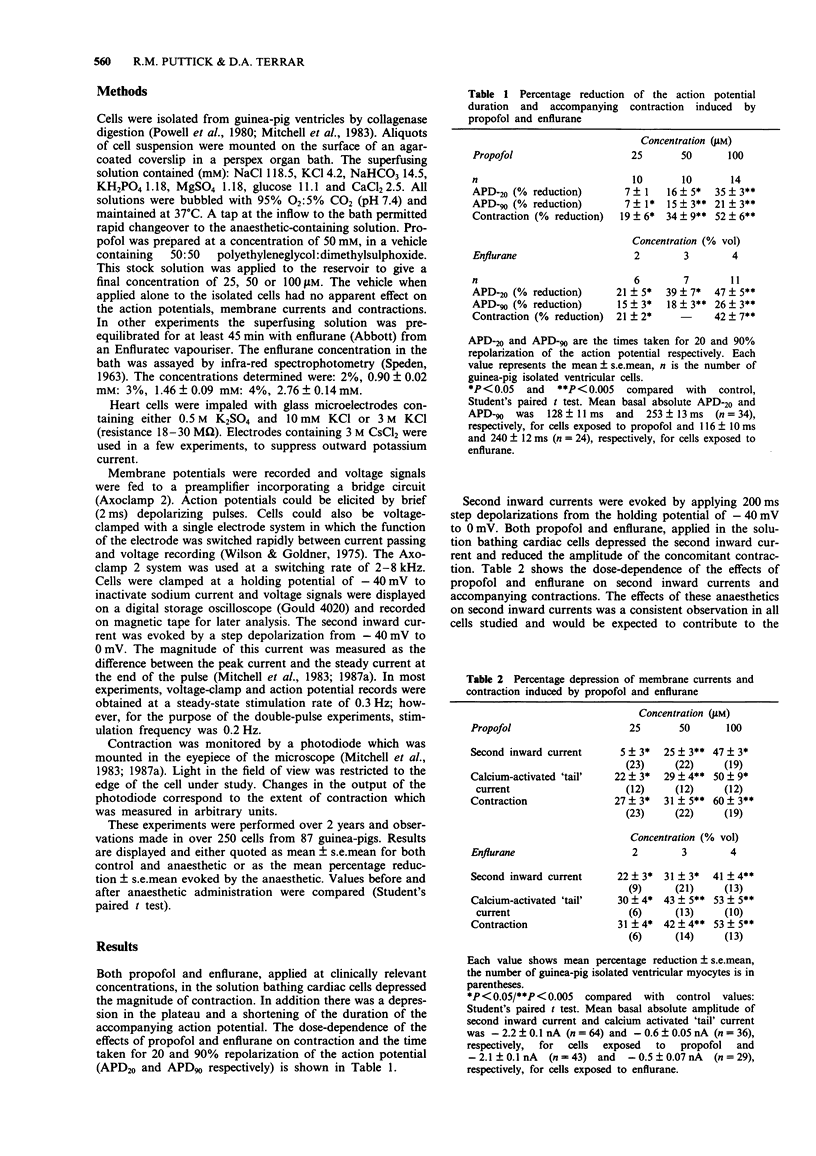
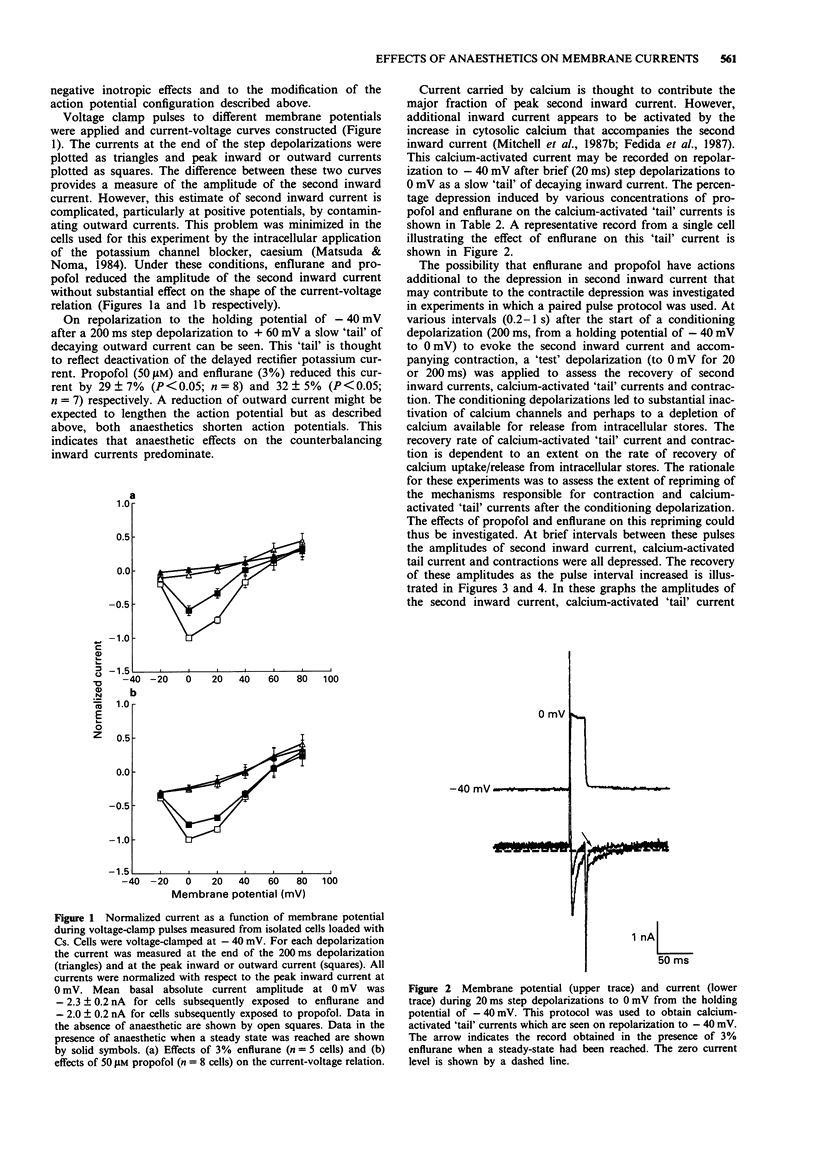
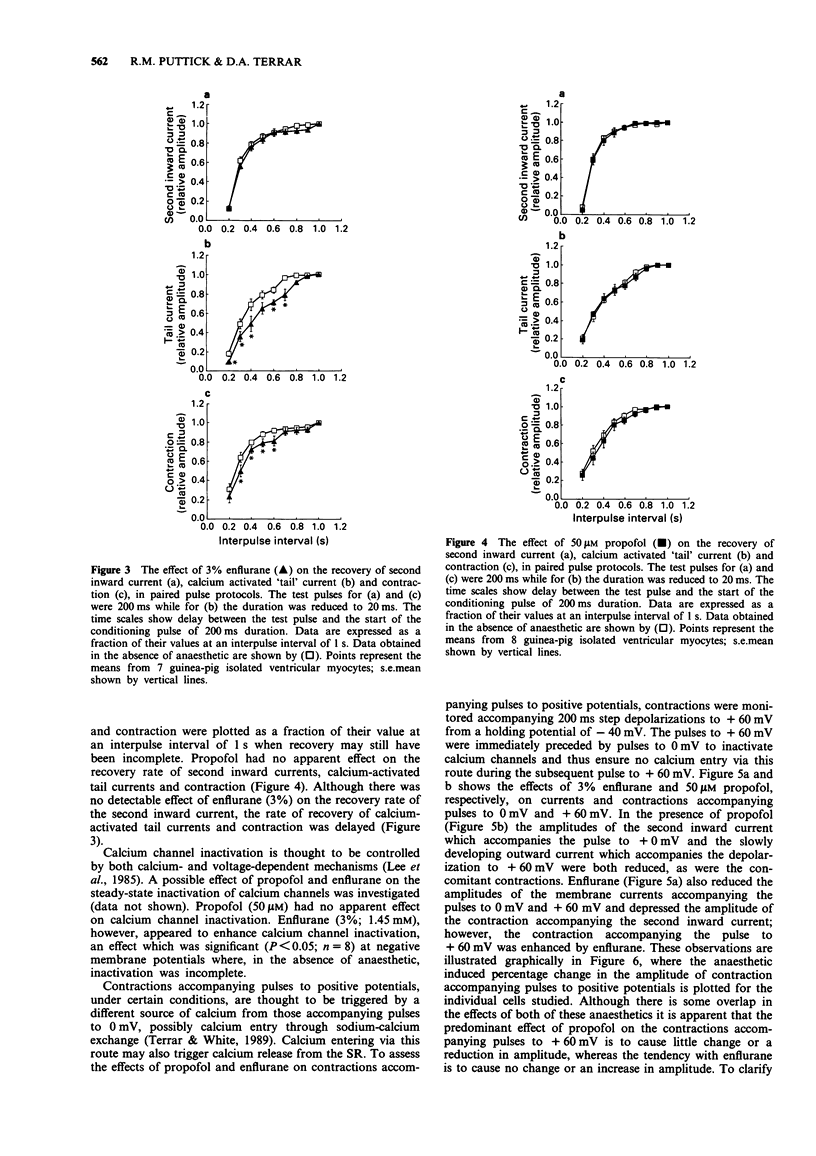
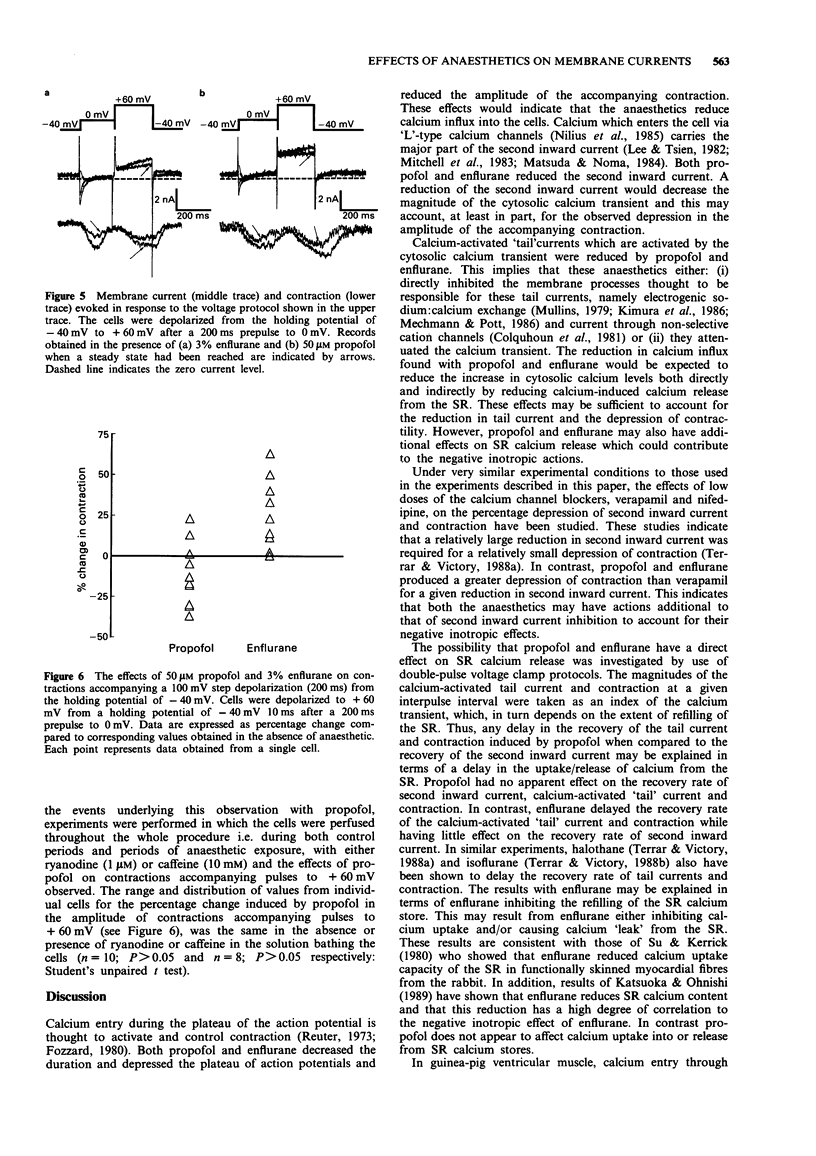
1. The effects of two general anaesthetics, propofol and enflurane, on electrical activity and contractions were investigated in single myocytes isolated from guinea-pig ventricles. 2. Propofol and enflurane depressed the plateau and shortened the duration of action potentials. 3. Under voltage-clamp conditions, propofol and enflurane reduced the amplitude of inward calcium current and of additional inward current activated by cytosolic calcium. 4. Contractions (measured with an optical technique) accompanying either action potentials or second inward currents (in response to depolarizations to 0 mV) were reduced by both anaesthetics. The mechanisms for calcium entry during contractions accompanying pulses to positive potentials such as +60 mV are thought to differ from those accompanying second inward currents which are evoked by pulses from -40 to 0 mV. Enflurane enhanced the amplitudes of contractions accompanying pulses to positive potentials; in contrast these contractions were depressed by propofol. 5. In experiments where recovery processes were investigated by use of pairs of voltage-clamp pulses with a variable interval between them, enflurane but not propofol slowed the recovery of contractions and calcium-activated 'tail' currents. These observations are consistent with the hypothesis that enflurane may impair calcium handling by the sarcoplasmic reticulum whereas propofol has little, if any, effect at this site. 6. In conclusion, the actions of propofol and enflurane on second inward currents contribute to their effects on action potentials and contraction. The negative inotropic effect of both anaesthetics may result partly from reduced calcium influx to trigger contraction, and for enflurane, partly from an impairment of calcium handling by the sarcoplasmic reticulum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Khudhairi D., Gordon G., Morgan M., Whitwam J. G. Acute cardiovascular changes following disoprofol. Effects in heavily sedated patients with coronary artery disease. Anaesthesia. 1982 Oct;37(10):1007–1010. doi: 10.1111/j.1365-2044.1982.tb01713.x. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Bosnjak Z. J., Supan F. D., Rusch N. J. The effects of halothane, enflurane, and isoflurane on calcium current in isolated canine ventricular cells. Anesthesiology. 1991 Feb;74(2):340–345. doi: 10.1097/00000542-199102000-00022. [DOI] [PubMed] [Google Scholar]
- Brill D. M., Fozzard H. A., Makielski J. C., Wasserstrom J. A. Effect of prolonged depolarizations on twitch tension and intracellular sodium activity in sheep cardiac Purkinje fibres. J Physiol. 1987 Mar;384:355–375. doi: 10.1113/jphysiol.1987.sp016459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. R., Jr, Crout J. R. A comparative study of the effects of five general anesthetics on myocardial contractility. I. Isometric conditions. Anesthesiology. 1971 Mar;34(3):236–245. doi: 10.1097/00000542-197103000-00007. [DOI] [PubMed] [Google Scholar]
- Calverley R. K., Smith N. T., Prys-Roberts C., Eger E. I., 2nd, Jones C. W. Cardiovascular effects of enflurane anesthesia during controlled ventilation in man. Anesth Analg. 1978 Nov-Dec;57(6):619–628. [PubMed] [Google Scholar]
- Claeys M. A., Gepts E., Camu F. Haemodynamic changes during anaesthesia induced and maintained with propofol. Br J Anaesth. 1988 Jan;60(1):3–9. doi: 10.1093/bja/60.1.3. [DOI] [PubMed] [Google Scholar]
- Coetzee A., Fourie P., Coetzee J., Badenhorst E., Rebel A., Bolliger C., Uebel R., Wium C., Lombard C. Effect of various propofol plasma concentrations on regional myocardial contractility and left ventricular afterload. Anesth Analg. 1989 Oct;69(4):473–483. [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- DeTraglia M. C., Komai H., Rusy B. F. Differential effects of inhalation anesthetics on myocardial potentiated-state contractions in vitro. Anesthesiology. 1988 Apr;68(4):534–540. doi: 10.1097/00000542-198804000-00010. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fedida D., Noble D., Shimoni Y., Spindler A. J. Inward current related to contraction in guinea-pig ventricular myocytes. J Physiol. 1987 Apr;385:565–589. doi: 10.1113/jphysiol.1987.sp016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild C. S., Serrao J. M. Cardiovascular effects of propofol in the anaesthetized dog. Br J Anaesth. 1989 Jul;63(1):87–92. doi: 10.1093/bja/63.1.87. [DOI] [PubMed] [Google Scholar]
- Herland J. S., Julian F. J., Stephenson D. G. Halothane increases Ca2+ efflux via Ca2+ channels of sarcoplasmic reticulum in chemically skinned rat myocardium. J Physiol. 1990 Jul;426:1–18. doi: 10.1113/jphysiol.1990.sp018124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuoka M., Kobayashi K., Ohnishi S. T. Volatile anesthetics decrease calcium content of isolated myocytes. Anesthesiology. 1989 Jun;70(6):954–960. doi: 10.1097/00000542-198906000-00012. [DOI] [PubMed] [Google Scholar]
- Katsuoka M., Ohnishi S. T. Inhalation anaesthetics decrease calcium content of cardiac sarcoplasmic reticulum. Br J Anaesth. 1989 Jun;62(6):669–673. doi: 10.1093/bja/62.6.669. [DOI] [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Reversal of current through calcium channels in dialysed single heart cells. Nature. 1982 Jun 10;297(5866):498–501. doi: 10.1038/297498a0. [DOI] [PubMed] [Google Scholar]
- Lunch C., 3rd, Vogel S., Pratila M. G., Sperelakis N. Enflurane depression of myocardial slow action potentials. J Pharmacol Exp Ther. 1982 Aug;222(2):405–409. [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Merin R. G., Kumazawa T., Honig C. R. Reversible interaction between halothane and Ca++ on cardiac actomyosin adenosine triphosphatase: mechanism and significance. J Pharmacol Exp Ther. 1974 Jul;190(1):1–14. [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Calcium-activated inward current and contraction in rat and guinea-pig ventricular myocytes. J Physiol. 1987 Oct;391:545–560. doi: 10.1113/jphysiol.1987.sp016755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Electrical activity and contraction in cells isolated from rat and guinea-pig ventricular muscle: a comparative study. J Physiol. 1987 Oct;391:527–544. doi: 10.1113/jphysiol.1987.sp016754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Murat I., Lechene P., Ventura-Clapier R. Effects of volatile anesthetics on mechanical properties of rat cardiac skinned fibers. Anesthesiology. 1990 Jul;73(1):73–81. doi: 10.1097/00000542-199007000-00012. [DOI] [PubMed] [Google Scholar]
- Murat I., Ventura-Clapier R., Vassort G. Halothane, enflurane, and isoflurane decrease calcium sensitivity and maximal force in detergent-treated rat cardiac fibers. Anesthesiology. 1988 Dec;69(6):892–899. doi: 10.1097/00000542-198812000-00015. [DOI] [PubMed] [Google Scholar]
- Nakao S., Hirata H., Kagawa Y. Effects of volatile anesthetics on cardiac calcium channels. Acta Anaesthesiol Scand. 1989 May;33(4):326–330. doi: 10.1111/j.1399-6576.1989.tb02917.x. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Pask H. T., England P. J., Prys-Roberts C. Effects of volatile inhalational anaesthetic agents on isolated bovine cardiac myofibrillar ATPase. J Mol Cell Cardiol. 1981 Mar;13(3):293–301. doi: 10.1016/0022-2828(81)90317-5. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Shimosato S., Sugai N., Iwatsuki N., Etsten B. E. The effect of ethrane on cardiac muscle mechanics. Anesthesiology. 1969 May;30(5):513–518. doi: 10.1097/00000542-196905000-00009. [DOI] [PubMed] [Google Scholar]
- Stephan H., Sonntag H., Schenk H. D., Kettler D., Khambatta H. J. Effects of propofol on cardiovascular dynamics, myocardial blood flow and myocardial metabolism in patients with coronary artery disease. Br J Anaesth. 1986 Sep;58(9):969–975. doi: 10.1093/bja/58.9.969. [DOI] [PubMed] [Google Scholar]
- Su J. Y., Kerrick W. G. Effects of enflurane on functionally skinned myocardial fibers from rabbits. Anesthesiology. 1980 May;52(5):385–389. doi: 10.1097/00000542-198005000-00002. [DOI] [PubMed] [Google Scholar]
- Terrar D. A., Victory J. G. Effects of halothane on membrane currents associated with contraction in single myocytes isolated from guinea-pig ventricle. Br J Pharmacol. 1988 Jun;94(2):500–508. doi: 10.1111/j.1476-5381.1988.tb11553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrar D. A., Victory J. G. Influence of halothane on contraction at positive membrane potentials in single cells isolated from guinea-pig ventricular muscle. Q J Exp Physiol. 1989 Mar;74(2):141–151. doi: 10.1113/expphysiol.1989.sp003251. [DOI] [PubMed] [Google Scholar]
- Terrar D. A., Victory J. G. Isoflurane depresses membrane currents associated with contraction in myocytes isolated from guinea-pig ventricle. Anesthesiology. 1988 Nov;69(5):742–749. doi: 10.1097/00000542-198811000-00017. [DOI] [PubMed] [Google Scholar]
- Terrar D. A., White E. Mechanisms and significance of calcium entry at positive membrane potentials in guinea-pig ventricular muscle cells. Q J Exp Physiol. 1989 Mar;74(2):121–139. doi: 10.1113/expphysiol.1989.sp003250. [DOI] [PubMed] [Google Scholar]
- Wheeler D. M., Rice R. T., Hansford R. G., Lakatta E. G. The effect of halothane on the free intracellular calcium concentration of isolated rat heart cells. Anesthesiology. 1988 Oct;69(4):578–583. doi: 10.1097/00000542-198810000-00019. [DOI] [PubMed] [Google Scholar]
- Wheeler D. M., Rice R. T., Lakatta E. G. The action of halothane on spontaneous contractile waves and stimulated contractions in isolated rat and dog heart cells. Anesthesiology. 1990 May;72(5):911–920. doi: 10.1097/00000542-199005000-00022. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]


