Abstract
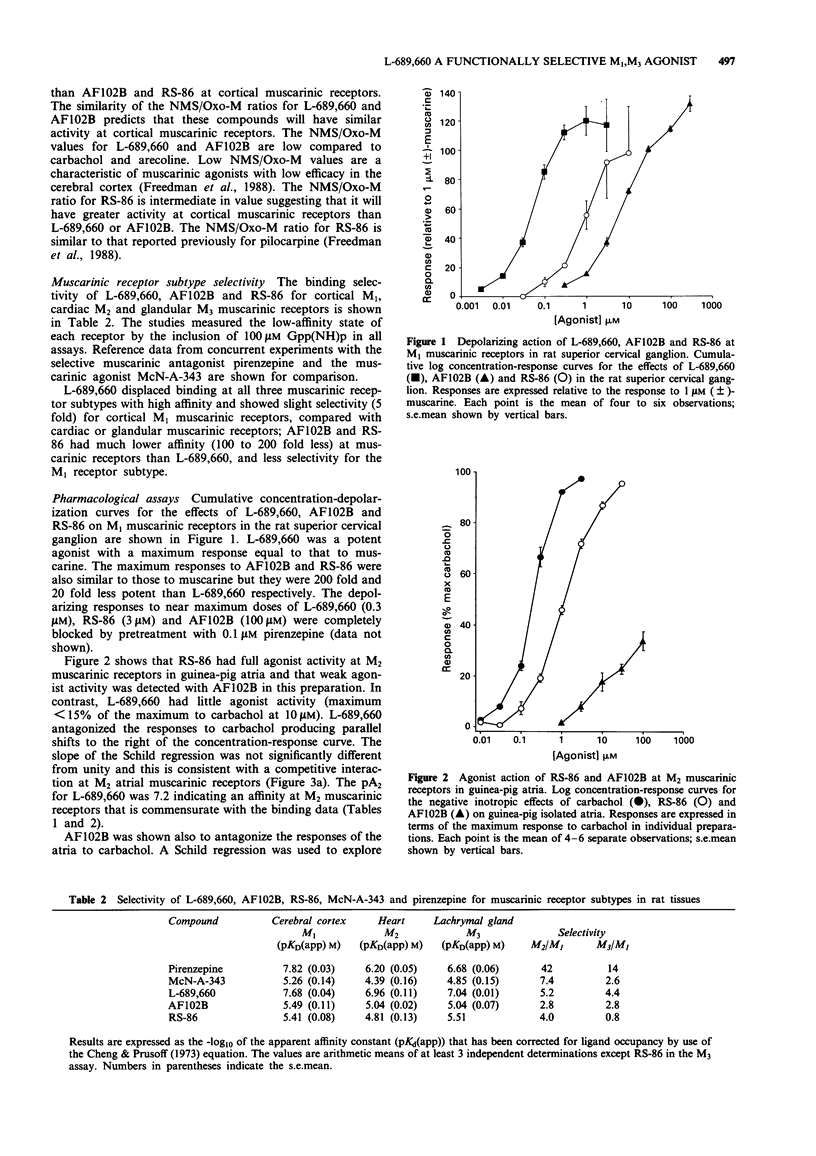
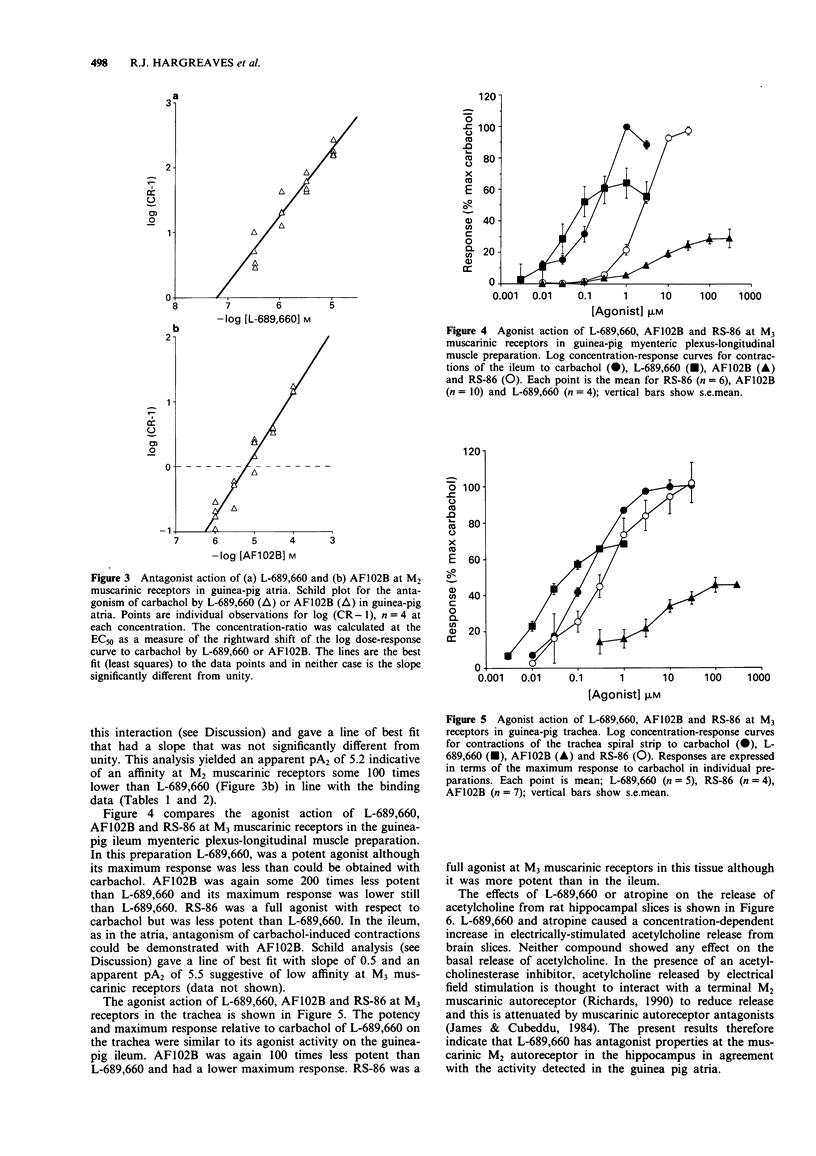
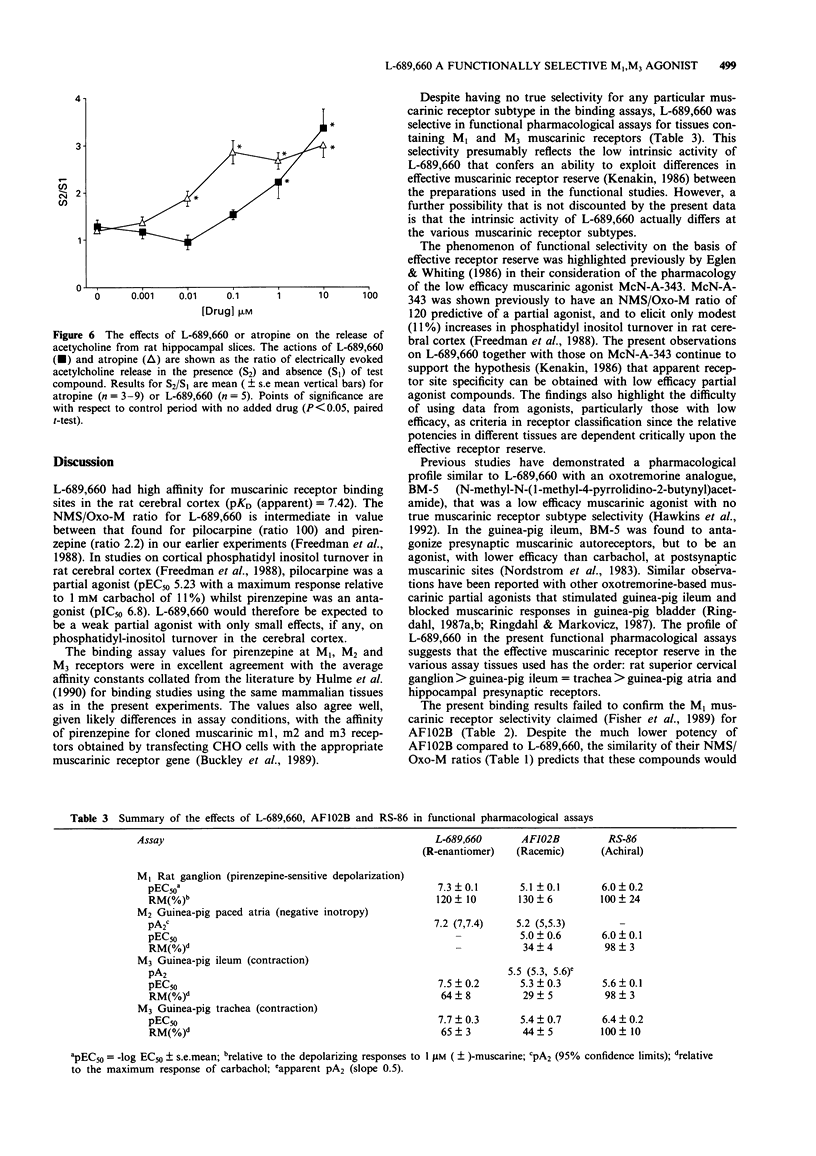
1. L-689,660, 1-azabicyclo[2.2.2]octane, 3-(6-chloropyrazinyl)maleate, a novel cholinomimetic, demonstrated high affinity binding (pKD (apparent) 7.42) at rat cerebral cortex muscarinic receptors. L-689,660 had a low ratio (34) of pKD (apparent) values for the displacement of binding of the antagonist ([3H]-N-methylscopolamine ([3H]-NMS) compared with the displacement of the agonist [3H]-oxotremorine-M ([3H]-Oxo-M), in rat cerebral cortex. Low NMS/Oxo-M ratios have been shown previously to be a characteristic of compounds that are low efficacy partial agonists with respect to stimulation of phosphatidyl inositol turnover in the cerebral cortex. 2. L-689,660 showed no muscarinic receptor subtype selectivity in radioligand binding assays but showed functional selectivity in pharmacological assays. At M1 muscarinic receptors in the rat superior cervical ganglion, L-689,660 was a potent (pEC50 7.3 +/- 0.2) full agonist in comparison with (+/-)-muscarine. At M3 receptors in the guinea-pig ileum myenteric plexus-longitudinal muscle or in trachea, L-689,660 was again a potent agonist (pEC50 7.5 +/- 0.2 and 7.7 +/- 0.3 respectively) but had a lower maximum response than carbachol. In contrast L-689,660 was an antagonist at M2 receptors in guinea-pig atria (pA2 7.2 (95% confidence limits 7, 7.4)) and at muscarinic autoreceptors in rat hippocampal slices. 3. The putative M1-selective muscarinic agonist, AF102B (cis-2-methylspiro-(1,3-oxathiolane 5,3')-quinuclidine hydrochloride) was found to have a profile similar to L-689,660 but had up to 100 times less affinity in binding and functional assays.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo D. M., Lapchak P. A., Robitaille Y., Gauthier S., Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer's disease. J Neurochem. 1988 Jun;50(6):1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Bartus R. T., Dean R. L., 3rd, Beer B., Lippa A. S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982 Jul 30;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Boddeke H. W., Buttini M. Pharmacological properties of cloned muscarinic receptors expressed in A9 L cells; comparison with in vitro models. Eur J Pharmacol. 1991 Sep 17;202(2):151–157. doi: 10.1016/0014-2999(91)90289-3. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Buckley N. J., Bonner T. I. The striatum and cerebral cortex express different muscarinic receptor mRNAs. FEBS Lett. 1988 Mar 28;230(1-2):90–94. doi: 10.1016/0014-5793(88)80648-3. [DOI] [PubMed] [Google Scholar]
- Brezenoff H. E., Giuliano R. Cardiovascular control by cholinergic mechanisms in the central nervous system. Annu Rev Pharmacol Toxicol. 1982;22:341–381. doi: 10.1146/annurev.pa.22.040182.002013. [DOI] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Brann M. R. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988 Dec;8(12):4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Davies P., Maloney A. J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976 Dec 25;2(8000):1403–1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Muscarinic receptor subtypes: a critique of the current classification and a proposal for a working nomenclature. J Auton Pharmacol. 1986 Dec;6(4):323–346. doi: 10.1111/j.1474-8673.1986.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Freedman S. B., Harley E. A., Iversen L. L. Relative affinities of drugs acting at cholinoceptors in displacing agonist and antagonist radioligands: the NMS/Oxo-M ratio as an index of efficacy at cortical muscarinic receptors. Br J Pharmacol. 1988 Feb;93(2):437–445. doi: 10.1111/j.1476-5381.1988.tb11451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S. B., Harley E. A., Patel S., Newberry N. R., Gilbert M. J., McKnight A. T., Tang J. K., Maguire J. J., Mudunkotuwa N. T., Baker R. A novel series of non-quaternary oxadiazoles acting as full agonists at muscarinic receptors. Br J Pharmacol. 1990 Nov;101(3):575–580. doi: 10.1111/j.1476-5381.1990.tb14123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. A., Enz A., Spiegel R. Muscarinic agonists for senile dementia: past experience and future trends. Trends Pharmacol Sci. 1989 Dec;Suppl:85–88. [PubMed] [Google Scholar]
- Hollander E., Mohs R. C., Davis K. L. Cholinergic approaches to the treatment of Alzheimer's disease. Br Med Bull. 1986 Jan;42(1):97–100. doi: 10.1093/oxfordjournals.bmb.a072106. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- James M. K., Cubeddu L. X. Frequency-dependent muscarinic receptor modulation of acetylcholine and dopamine release from rabbit striatum. J Pharmacol Exp Ther. 1984 Apr;229(1):98–104. [PubMed] [Google Scholar]
- Kenakin T. Drugs and receptors. An overview of the current state of knowledge. Drugs. 1990 Nov;40(5):666–687. doi: 10.2165/00003495-199040050-00003. [DOI] [PubMed] [Google Scholar]
- Mash D. C., Flynn D. D., Potter L. T. Loss of M2 muscarine receptors in the cerebral cortex in Alzheimer's disease and experimental cholinergic denervation. Science. 1985 May 31;228(4703):1115–1117. doi: 10.1126/science.3992249. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Priestley T. Pharmacological differences between two muscarinic responses of the rat superior cervical ganglion in vitro. Br J Pharmacol. 1987 Dec;92(4):817–826. doi: 10.1111/j.1476-5381.1987.tb11386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström O., Alberts P., Westlind A., Undén A., Bartfai T. Presynaptic antagonist-postsynaptic agonist at muscarinic cholinergic synapses. N-methyl-N-(1-methyl-4-pyrrolidino-2-butynyl)acetamide. Mol Pharmacol. 1983 Jul;24(1):1–5. [PubMed] [Google Scholar]
- Nordström O., Bartfai T. Muscarinic autoreceptor regulates acetylcholine release in rat hippocampus: in vitro evidence. Acta Physiol Scand. 1980 Apr;108(4):347–353. doi: 10.1111/j.1748-1716.1980.tb06543.x. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Bolliger G., Closse A., Enz A., Gmelin G., Malanowski J. The pharmacological assessment of RS 86 (2-ethyl-8-methyl-2,8-diazaspiro-[4,5]-decan-1,3-dion hydrobromide). A potent, specific muscarinic acetylcholine receptor agonist. Eur J Pharmacol. 1986 Jun 5;125(1):45–62. doi: 10.1016/0014-2999(86)90082-8. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Mengod G., Vilaró M. T., Wiederhold K. H., Boddeke H., Alvarez F. J., Chinaglia G., Probst A. Cholinergic receptors in the rat and human brain: microscopic visualization. Prog Brain Res. 1990;84:243–253. doi: 10.1016/s0079-6123(08)60909-7. [DOI] [PubMed] [Google Scholar]
- Pazos A., Wiederhold K. H., Palacios J. M. Central pressor effects induced by muscarinic receptor agonists: evidence for a predominant role of the M2 receptor subtype. Eur J Pharmacol. 1986 Jun 5;125(1):63–70. doi: 10.1016/0014-2999(86)90083-x. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Perry R. H., Blessed G., Tomlinson B. E. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977 Jan 22;1(8004):189–189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- Perry E. K. The cholinergic hypothesis--ten years on. Br Med Bull. 1986 Jan;42(1):63–69. doi: 10.1093/oxfordjournals.bmb.a072100. [DOI] [PubMed] [Google Scholar]
- Probst A., Cortés R., Ulrich J., Palacios J. M. Differential modification of muscarinic cholinergic receptors in the hippocampus of patients with Alzheimer's disease: an autoradiographic study. Brain Res. 1988 May 31;450(1-2):190–201. doi: 10.1016/0006-8993(88)91558-2. [DOI] [PubMed] [Google Scholar]
- Richards M. H. Rat hippocampal muscarinic autoreceptors are similar to the M2 (cardiac) subtype: comparison with hippocampal M1, atrial M2 and ileal M3 receptors. Br J Pharmacol. 1990 Apr;99(4):753–761. doi: 10.1111/j.1476-5381.1990.tb13002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley R. M., Aitken D. M., Baker H. F. Learning about rules but not about reward is impaired following lesions of the cholinergic projection to the hippocampus. Brain Res. 1989 Nov 20;502(2):306–318. doi: 10.1016/0006-8993(89)90626-4. [DOI] [PubMed] [Google Scholar]
- Ridley R. M., Baker H. F., Drewett B., Johnson J. A. Effects of ibotenic acid lesions of the basal forebrain on serial reversal learning in marmosets. Psychopharmacology (Berl) 1985;86(4):438–443. doi: 10.1007/BF00427905. [DOI] [PubMed] [Google Scholar]
- Ridley R. M., Murray T. K., Johnson J. A., Baker H. F. Learning impairment following lesion of the basal nucleus of Meynert in the marmoset: modification by cholinergic drugs. Brain Res. 1986 Jun 18;376(1):108–116. doi: 10.1016/0006-8993(86)90904-2. [DOI] [PubMed] [Google Scholar]
- Ringdahl B., Markowicz M. E. Muscarinic and antimuscarinic activity of acetamides related to oxotremorine in the guinea pig urinary bladder. J Pharmacol Exp Ther. 1987 Mar;240(3):789–794. [PubMed] [Google Scholar]
- Ringdahl B. Structural requirements for affinity and efficacy of N-(4-amino-2-butynyl)succinimides at muscarinic receptors in the guinea-pig ileum and urinary bladder. Eur J Pharmacol. 1987 Aug 4;140(1):13–23. doi: 10.1016/0014-2999(87)90628-5. [DOI] [PubMed] [Google Scholar]
- Sapru H. N. Cholinergic mechanisms subserving cardiovascular function in the medulla and spinal cord. Prog Brain Res. 1989;81:171–179. doi: 10.1016/s0079-6123(08)62007-5. [DOI] [PubMed] [Google Scholar]
- Sims N. R., Bowen D. M., Allen S. J., Smith C. C., Neary D., Thomas D. J., Davison A. N. Presynaptic cholinergic dysfunction in patients with dementia. J Neurochem. 1983 Feb;40(2):503–509. doi: 10.1111/j.1471-4159.1983.tb11311.x. [DOI] [PubMed] [Google Scholar]


