Abstract
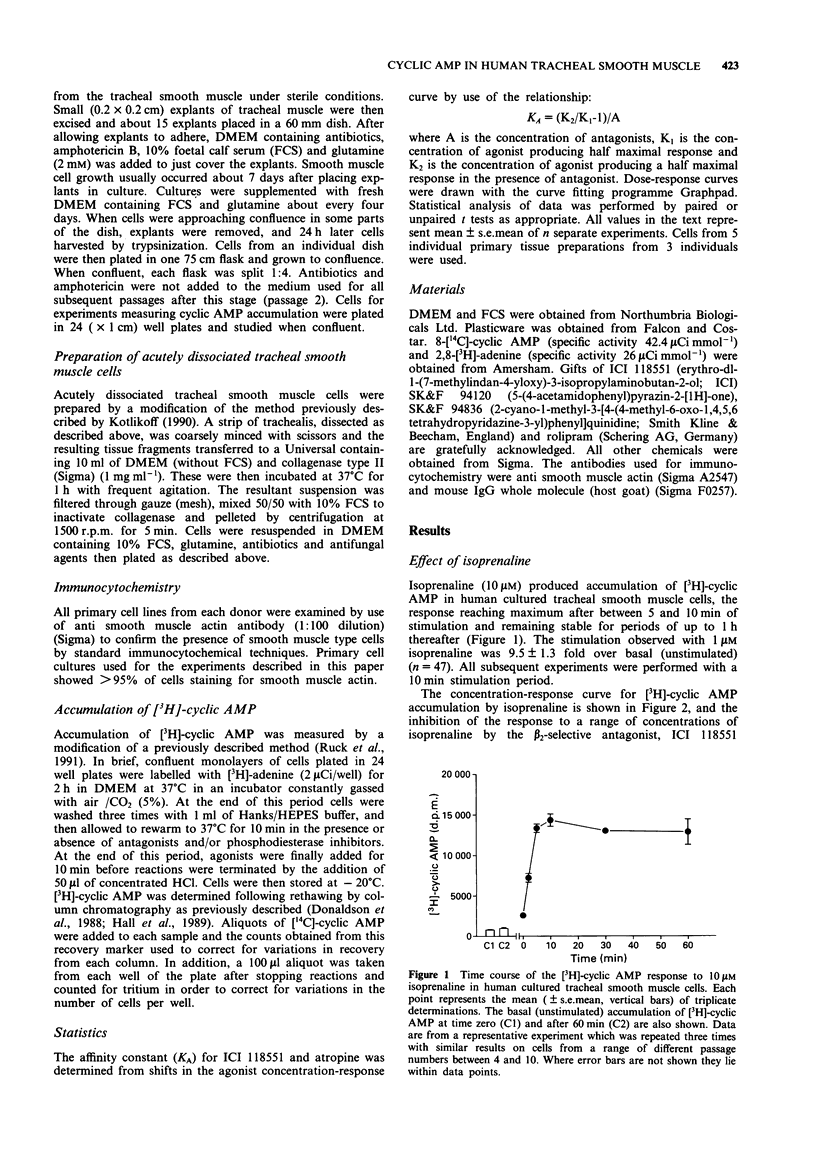
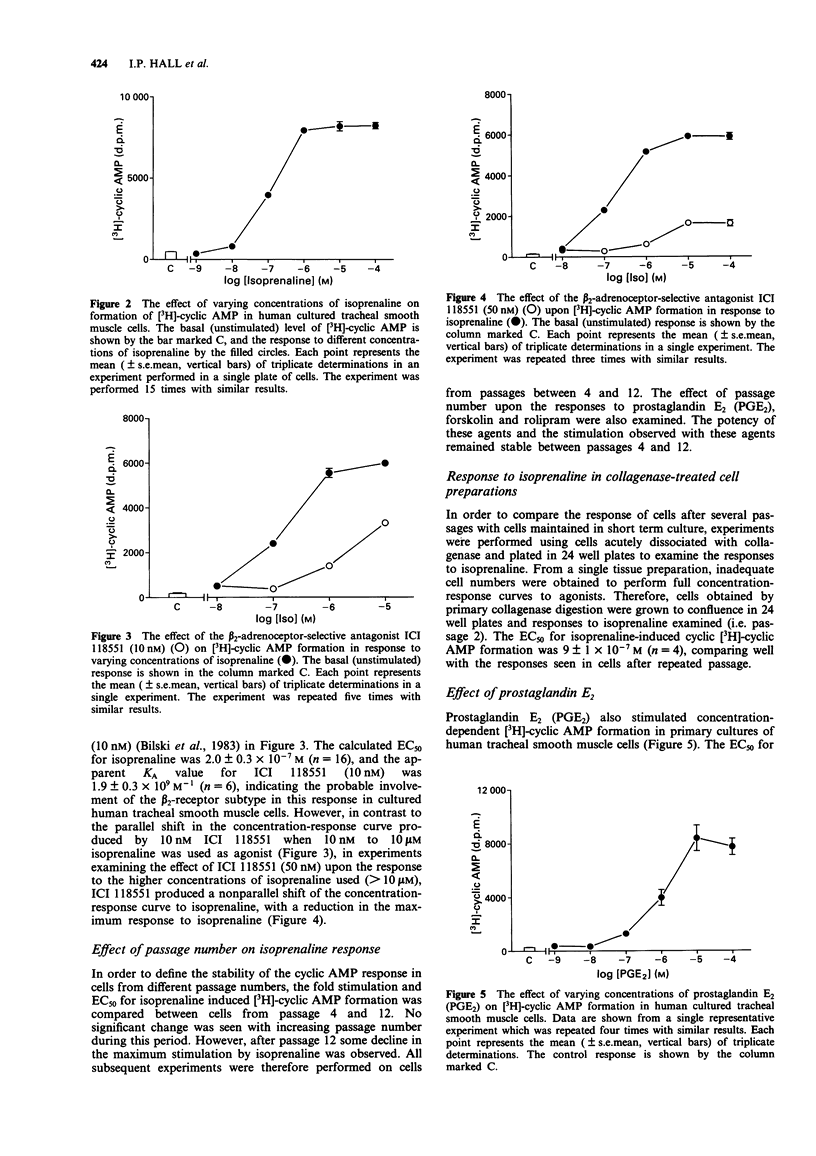
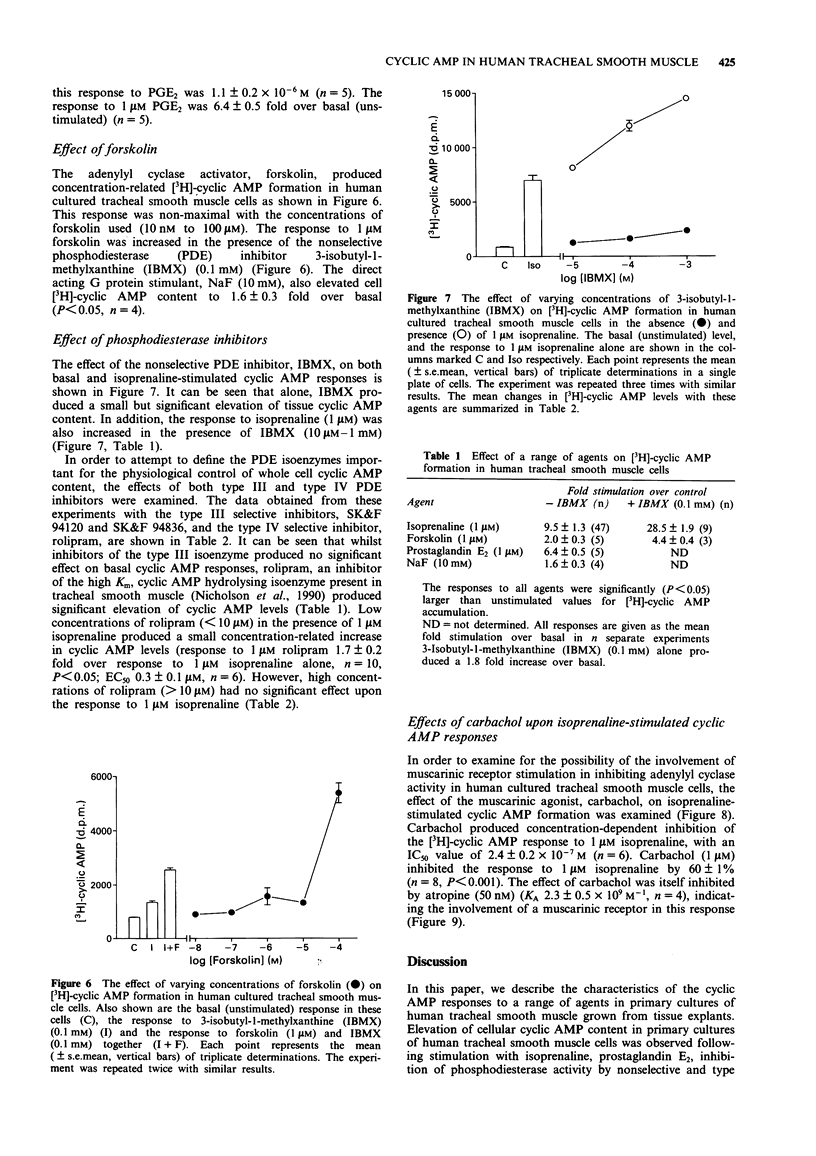
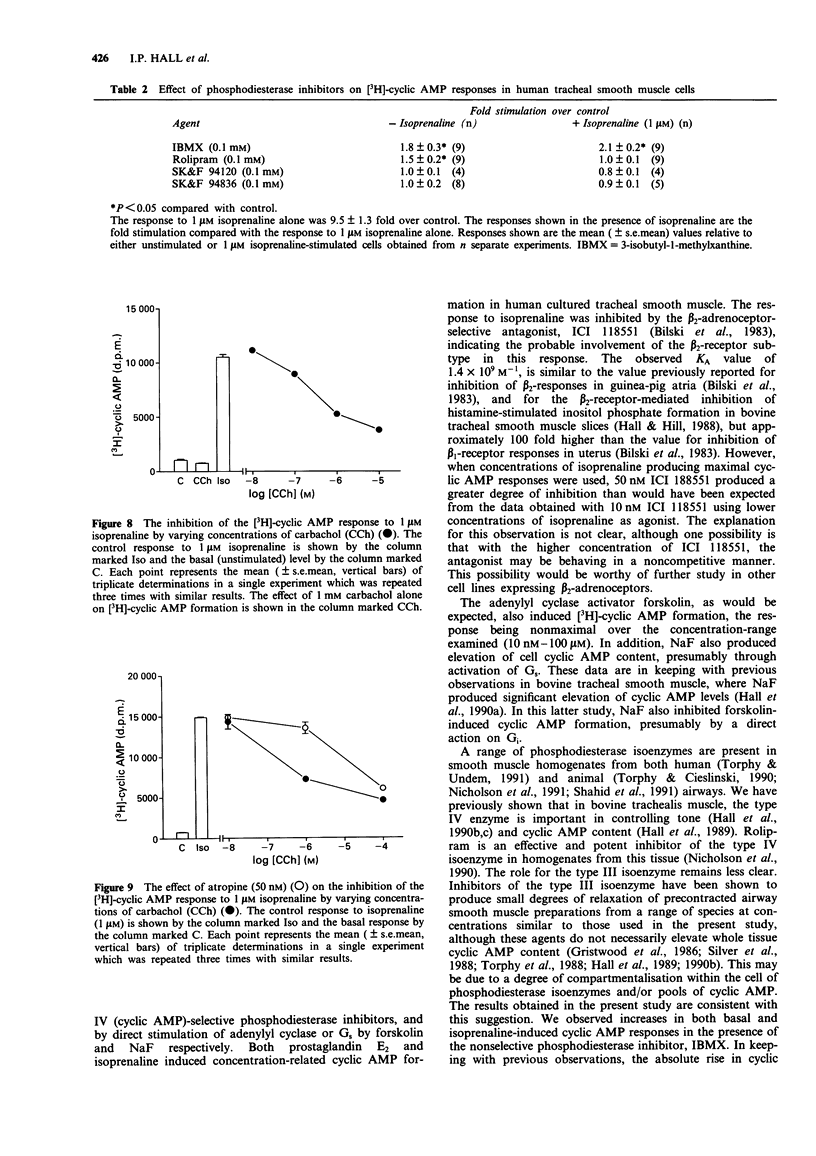
1. [3H]-adenosine 3':5'-cyclic monophosphate ([3H]-cyclic AMP) responses were studied in primary cultures of human tracheal smooth muscle cells derived from explants of human trachealis muscle and in short term cultures of acutely dissociated trachealis cells. 2. Isoprenaline induced concentration-dependent [3H]-cyclic AMP formation with an EC50 of 0.2 microM. The response to 10 microM isoprenaline reached a maximum after 5-10 min stimulation and remained stable for periods of up to 1 h. After 10 min stimulation, 1 microM isoprenaline produced a 9.5 fold increase over basal [3H]-cyclic AMP levels. The response to isoprenaline was inhibited by ICI 118551 (10 nM), (apparent KA 1.9 x 10(9) M-1) indicating the probable involvement of a beta 2-adrenoceptor in this response in human cultured tracheal smooth muscle cells. However, with 50 nM ICI 118551 there was a reduction in the maximum response to isoprenaline. Prostaglandin E2 also produced concentration-dependent [3H]-cyclic AMP formation (EC50 0.7 microM, response to 1 microM PGE2 6.4 fold over basal). 3. Forskolin (1 nM - 100 microM) induced concentration-dependent [3H]-cyclic AMP formation in these cells. A 1.6 fold (over basal) response was also observed following stimulation with NaF (10 mM). 4. The nonselective phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) (0.1 mM) and the type IV, cyclic AMP selective, phosphodiesterase inhibitor rolipram (0.1 mM) both elevated basal [3H]-cyclic AMP levels by 1.8 and 1.5 fold respectively. IBMX (1-100 microM) and low concentrations of rolipram (< 10 microM), also potentiated the response to 1 microM isoprenaline.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilski A. J., Halliday S. E., Fitzgerald J. D., Wale J. L. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551). J Cardiovasc Pharmacol. 1983 May-Jun;5(3):430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J., Brown A. M. Kinetic studies on the mechanism by which histamine H1 receptors potentiate cyclic AMP accumulation in guinea pig cerebral cortical slices. Mol Pharmacol. 1988 Jun;33(6):626–633. [PubMed] [Google Scholar]
- Feth F., Rascher W., Michel M. C. Neuropeptide Y (NPY) receptors in HEL cells: comparison of binding and functional parameters for full and partial agonists and a non-peptide antagonist. Br J Pharmacol. 1992 Jan;105(1):71–76. doi: 10.1111/j.1476-5381.1992.tb14212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Sumimoto K., Itoh T., Suzuki H., Kuriyama H. Relaxing actions of procaterol, a beta 2-adrenoceptor stimulant, on smooth muscle cells of the dog trachea. Br J Pharmacol. 1988 Jan;93(1):199–209. doi: 10.1111/j.1476-5381.1988.tb11422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristwood R. W., Eden R. J., Owen D. A., Taylor E. M. Pharmacological studies with SK&F 94120, a novel positive inotropic agent with vasodilator activity. J Pharm Pharmacol. 1986 Jun;38(6):452–459. doi: 10.1111/j.2042-7158.1986.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Inhibition of histamine-stimulated inositol phospholipid hydrolysis by agents which increase cyclic AMP levels in bovine tracheal smooth muscle. Br J Pharmacol. 1989 Jun;97(2):603–613. doi: 10.1111/j.1476-5381.1989.tb11992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Modulation of carbachol-induced inositol phosphate formation in bovine tracheal smooth muscle by cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol. 1990 Apr 15;39(8):1357–1363. doi: 10.1016/0006-2952(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Modulation of fluoroaluminate-induced inositol phosphate formation by increases in tissue cyclic AMP content in bovine tracheal smooth muscle. Br J Pharmacol. 1990 Jul;100(3):646–650. doi: 10.1111/j.1476-5381.1990.tb15861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. P., Hill S. J. Beta-adrenoceptor stimulation inhibits histamine-stimulated inositol phospholipid hydrolysis in bovine tracheal smooth muscle. Br J Pharmacol. 1988 Dec;95(4):1204–1212. doi: 10.1111/j.1476-5381.1988.tb11757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Connell M. J., Ferguson E. W., Wallace A. M., Gordon R. J., Pagani E. D., Silver P. J. Role of low Km cyclic AMP phosphodiesterase inhibition in tracheal relaxation and bronchodilation in the guinea pig. J Pharmacol Exp Ther. 1989 Oct;251(1):199–206. [PubMed] [Google Scholar]
- Jones C. A., Madison J. M., Tom-Moy M., Brown J. K. Muscarinic cholinergic inhibition of adenylate cyclase in airway smooth muscle. Am J Physiol. 1987 Jul;253(1 Pt 1):C97–104. doi: 10.1152/ajpcell.1987.253.1.C97. [DOI] [PubMed] [Google Scholar]
- Kotlikoff M. I. Potassium currents in canine airway smooth muscle cells. Am J Physiol. 1990 Dec;259(6 Pt 1):L384–L395. doi: 10.1152/ajplung.1990.259.6.L384. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Mueller E., van Breemen C. Role of intracellular Ca2+ sequestration in beta-adrenergic relaxation of a smooth muscle. Nature. 1979 Oct 25;281(5733):682–683. doi: 10.1038/281682a0. [DOI] [PubMed] [Google Scholar]
- Nicholson C. D., Challiss R. A., Shahid M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol Sci. 1991 Jan;12(1):19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- Reeves M. L., Leigh B. K., England P. J. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987 Jan 15;241(2):535–541. doi: 10.1042/bj2410535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruck A., Kendall D. A., Hill S. J. Alpha- and beta-adrenoceptor regulation of cyclic AMP accumulation in cultured rat astrocytes. A comparison of primary protoplasmic and mixed fibrous/protoplasmic astroglial cultures. Biochem Pharmacol. 1991 Jun 21;42(1):59–69. doi: 10.1016/0006-2952(91)90681-t. [DOI] [PubMed] [Google Scholar]
- Sankary R. M., Jones C. A., Madison J. M., Brown J. K. Muscarinic cholinergic inhibition of cyclic AMP accumulation in airway smooth muscle. Role of a pertussis toxin-sensitive protein. Am Rev Respir Dis. 1988 Jul;138(1):145–150. doi: 10.1164/ajrccm/138.1.145. [DOI] [PubMed] [Google Scholar]
- Shahid M., van Amsterdam R. G., de Boer J., ten Berge R. E., Nicholson C. D., Zaagsma J. The presence of five cyclic nucleotide phosphodiesterase isoenzyme activities in bovine tracheal smooth muscle and the functional effects of selective inhibitors. Br J Pharmacol. 1991 Oct;104(2):471–477. doi: 10.1111/j.1476-5381.1991.tb12453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. J., Hamel L. T., Perrone M. H., Bentley R. G., Bushover C. R., Evans D. B. Differential pharmacologic sensitivity of cyclic nucleotide phosphodiesterase isozymes isolated from cardiac muscle, arterial and airway smooth muscle. Eur J Pharmacol. 1988 May 20;150(1-2):85–94. doi: 10.1016/0014-2999(88)90753-4. [DOI] [PubMed] [Google Scholar]
- Silver P. J., Stull J. T. Regulation of myosin light chain and phosphorylase phosphorylation in tracheal smooth muscle. J Biol Chem. 1982 Jun 10;257(11):6145–6150. [PubMed] [Google Scholar]
- Torphy T. J., Burman M., Huang L. B., Horohonich S., Cieslinski L. B. Regulation of cAMP content and cAMP-dependent protein kinase activity in airway smooth muscle. Prog Clin Biol Res. 1987;245:263–275. [PubMed] [Google Scholar]
- Torphy T. J., Burman M., Huang L. B., Tucker S. S. Inhibition of the low km cyclic AMP phosphodiesterase in intact canine trachealis by SK&F 94836: mechanical and biochemical responses. J Pharmacol Exp Ther. 1988 Sep;246(3):843–850. [PubMed] [Google Scholar]
- Torphy T. J., Cieslinski L. B. Characterization and selective inhibition of cyclic nucleotide phosphodiesterase isozymes in canine tracheal smooth muscle. Mol Pharmacol. 1990 Feb;37(2):206–214. [PubMed] [Google Scholar]
- Torphy T. J., Undem B. J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991 Jul;46(7):512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. M., Chou S. P., Sung T. C. Muscarinic receptor subtypes coupled to generation of different second messengers in isolated tracheal smooth muscle cells. Br J Pharmacol. 1991 Nov;104(3):613–618. doi: 10.1111/j.1476-5381.1991.tb12478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongste J. C., Mons H., Bonta I. L., Kerrebijn K. F. Relaxation of human peripheral airway smooth muscle in vitro does not correlate with severity of chronic airflow limitation in vivo. Pulm Pharmacol. 1989;2(2):75–79. doi: 10.1016/0952-0600(89)90027-6. [DOI] [PubMed] [Google Scholar]



