Abstract
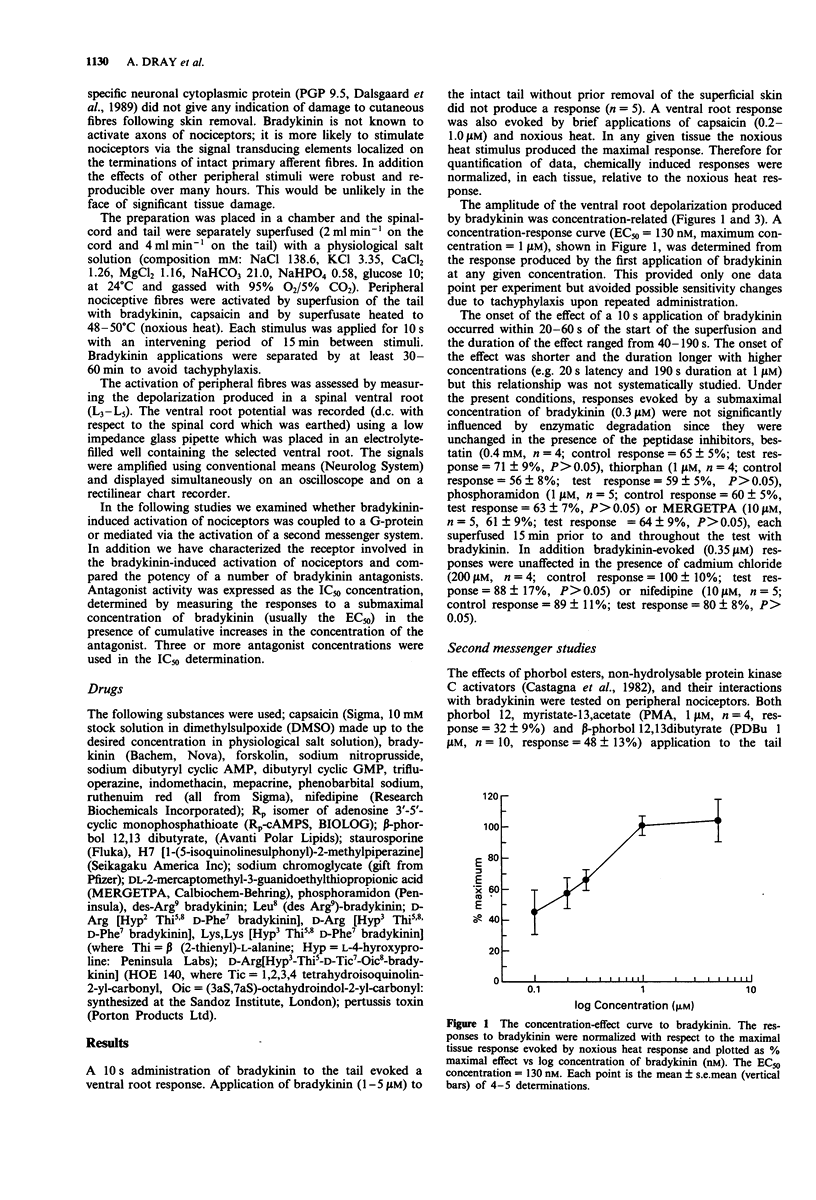
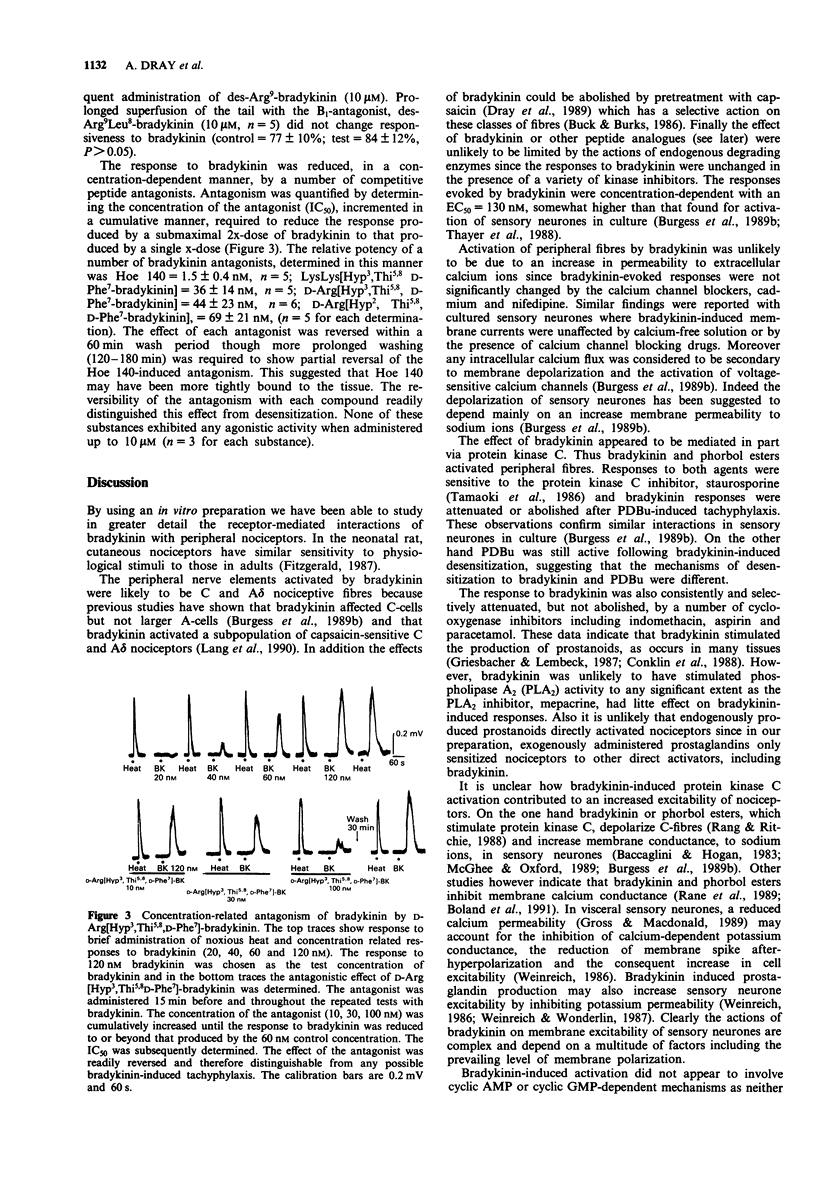
1. The effects of bradykinin on nociceptors have been characterized on a preparation of the neonatal rat spinal cord with functionally connected tail maintained in vitro. Administration of bradykinin to the tail activated capsaicin-sensitive peripheral fibres and evoked a concentration-dependent (EC50 = 130 nM) depolarization recorded from a spinal ventral root (L3-L5). 2. The response to bradykinin was unaffected by the peptidase inhibitors, bestatin (0.4 mM), thiorphan (1 microM), phosphoramidon (1 microM) and MERGETPA (10 microM) or by the presence of calcium blocking agents, cadmium (200 microM) and nifedipine (10 microM). 3. Inhibition of cyclo-oxygenase with indomethacin (1-5 microM), aspirin (1-10 microM) and paracetamol (10-50 microM) consistently attenuated responses to bradykinin. 4. The effect of bradykinin was mimicked by the phorbol ester PDBu, an activator of protein kinase C. The response to bradykinin was attenuated following desensitization to PDBu but desensitization to bradykinin did not induce a cross-desensitization to PDBu. The protein kinase C inhibitor staurosporine (10-500 nM) consistently attenuated the effects of PDBu and bradykinin. 5. Bradykinin responses were reversibly enhanced by dibutyryl cyclic AMP (100 microM). However dibutyryl cyclic GMP (0.5 mM) and nitroprusside (10 microM) produced prolonged block of responsiveness to bradykinin. Prolonged superfusion with pertussis toxin did not affect responses to bradykinin. 6. The B1-receptor agonist des Arg9-bradykinin (10-100 microM) was ineffective alone or after prolonged exposure of the tail to lipopolysaccharide (100 ng ml-1) or epidermal growth factor (100 ng ml-1) to induce B1 receptors.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccaglini P. I., Hogan P. G. Some rat sensory neurons in culture express characteristics of differentiated pain sensory cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):594–598. doi: 10.1073/pnas.80.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974 Mar 11;347(3):209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- Boland L. M., Allen A. C., Dingledine R. Inhibition by bradykinin of voltage-activated barium current in a rat dorsal root ganglion cell line: role of protein kinase C. J Neurosci. 1991 Apr;11(4):1140–1149. doi: 10.1523/JNEUROSCI.11-04-01140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho L. H., Rothermel J. D., Coombs R. V., Jastorff B. cAMP analog antagonists of cAMP action. Methods Enzymol. 1988;159:159–172. doi: 10.1016/0076-6879(88)59017-1. [DOI] [PubMed] [Google Scholar]
- Bouthillier J., Deblois D., Marceau F. Studies on the induction of pharmacological responses to des-Arg9-bradykinin in vitro and in vivo. Br J Pharmacol. 1987 Oct;92(2):257–264. doi: 10.1111/j.1476-5381.1987.tb11319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Inositol 1,4,5-trisphosphate and diacylglycerol mimic bradykinin effects on mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:185–207. doi: 10.1113/jphysiol.1988.sp016995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Burch R. M., DeHaas C. A bradykinin antagonist inhibits carrageenan edema in rats. Naunyn Schmiedebergs Arch Pharmacol. 1990 Aug;342(2):189–193. doi: 10.1007/BF00166963. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Mullaney I., McNeill M., Coote P. R., Minhas A., Wood J. N. Activation of guanylate cyclase by bradykinin in rat sensory neurones is mediated by calcium influx: possible role of the increase in cyclic GMP. J Neurochem. 1989 Oct;53(4):1212–1218. doi: 10.1111/j.1471-4159.1989.tb07417.x. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Mullaney I., McNeill M., Dunn P. M., Rang H. P. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci. 1989 Sep;9(9):3314–3325. doi: 10.1523/JNEUROSCI.09-09-03314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess J. F. On Some Aspects of the Cultural Study of the Ringworm Fungi. Can Med Assoc J. 1925 Oct;15(10):1003–1007. [PMC free article] [PubMed] [Google Scholar]
- Chauhan V. P., Brockerhoff H. Phenobarbital competes with diacylglycerol for protein kinase C. Life Sci. 1987 Jan 5;40(1):89–93. doi: 10.1016/0024-3205(87)90256-6. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Burch R. M., Steranka L. R., Axelrod J. Distinct bradykinin receptors mediate stimulation of prostaglandin synthesis by endothelial cells and fibroblasts. J Pharmacol Exp Ther. 1988 Feb;244(2):646–649. [PubMed] [Google Scholar]
- Costello A. H., Hargreaves K. M. Suppression of carrageenan-induced hyperalgesia, hyperthermia and edema by a bradykinin antagonist. Eur J Pharmacol. 1989 Nov 21;171(2-3):259–263. doi: 10.1016/0014-2999(89)90118-0. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Rydh M., Haegerstrand A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry. 1989;92(5):385–390. doi: 10.1007/BF00492495. [DOI] [PubMed] [Google Scholar]
- Dixon M., Jackson D. M., Richards I. M. The action of sodium cromoglycate on 'C' fibre endings in the dog lung. Br J Pharmacol. 1980 Sep;70(1):11–13. doi: 10.1111/j.1476-5381.1980.tb10898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Bettaney J., Forster P. Actions of capsaicin on peripheral nociceptors of the neonatal rat spinal cord-tail in vitro: dependence of extracellular ions and independence of second messengers. Br J Pharmacol. 1990 Nov;101(3):727–733. doi: 10.1111/j.1476-5381.1990.tb14148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Bettaney J., Forster P. Capsaicin desensitization of peripheral nociceptive fibres does not impair sensitivity to other noxious stimuli. Neurosci Lett. 1989 Apr 24;99(1-2):50–54. doi: 10.1016/0304-3940(89)90263-2. [DOI] [PubMed] [Google Scholar]
- Dray A., Bettaney J., Forster P., Perkins M. N. Activation of a bradykinin receptor in peripheral nerve and spinal cord in the neonatal rat in vitro. Br J Pharmacol. 1988 Dec;95(4):1008–1010. doi: 10.1111/j.1476-5381.1988.tb11732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Bettaney J., Forster P., Perkins M. N. Bradykinin-induced stimulation of afferent fibres is mediated through protein kinase C. Neurosci Lett. 1988 Sep 12;91(3):301–307. doi: 10.1016/0304-3940(88)90697-0. [DOI] [PubMed] [Google Scholar]
- Dray A., Forbes C. A., Burgess G. M. Ruthenium red blocks the capsaicin-induced increase in intracellular calcium and activation of membrane currents in sensory neurones as well as the activation of peripheral nociceptors in vitro. Neurosci Lett. 1990 Mar 2;110(1-2):52–59. doi: 10.1016/0304-3940(90)90786-9. [DOI] [PubMed] [Google Scholar]
- Dunn P. M., Coote P. R., Wood J. N., Burgess G. M., Rang H. P. Bradykinin evoked depolarization of a novel neuroblastoma x DRG neurone hybrid cell line (ND7/23). Brain Res. 1991 Apr 5;545(1-2):80–86. doi: 10.1016/0006-8993(91)91272-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. J Physiol. 1987 Feb;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M., Mense S. Muscle receptors with group IV afferent fibres responding to application of bradykinin. Brain Res. 1975 Jul 18;92(3):369–383. doi: 10.1016/0006-8993(75)90323-6. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Dixon C. M., Cuss F. M., Barnes P. J. Bradykinin-induced bronchoconstriction in humans. Mode of action. Am Rev Respir Dis. 1987 Jan;135(1):176–180. doi: 10.1164/arrd.1987.135.1.176. [DOI] [PubMed] [Google Scholar]
- Gammon C. M., Allen A. C., Morell P. Bradykinin stimulates phosphoinositide hydrolysis and mobilization of arachidonic acid in dorsal root ganglion neurons. J Neurochem. 1989 Jul;53(1):95–101. doi: 10.1111/j.1471-4159.1989.tb07299.x. [DOI] [PubMed] [Google Scholar]
- Grega D. S., Macdonald R. L. Activators of adenylate cyclase and cyclic AMP prolong calcium-dependent action potentials of mouse sensory neurons in culture by reducing a voltage-dependent potassium conductance. J Neurosci. 1987 Mar;7(3):700–707. doi: 10.1523/JNEUROSCI.07-03-00700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbacher T., Lembeck F. Effect of bradykinin antagonists on bradykinin-induced plasma extravasation, venoconstriction, prostaglandin E2 release, nociceptor stimulation and contraction of the iris sphincter muscle in the rabbit. Br J Pharmacol. 1987 Oct;92(2):333–340. doi: 10.1111/j.1476-5381.1987.tb11328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., MacDonald R. L. Activators of protein kinase C selectively enhance inactivation of a calcium current component of cultured sensory neurons in a pertussis toxin-sensitive manner. J Neurophysiol. 1989 Jun;61(6):1259–1269. doi: 10.1152/jn.1989.61.6.1259. [DOI] [PubMed] [Google Scholar]
- Haley J. E., Dickenson A. H., Schachter M. Electrophysiological evidence for a role of bradykinin in chemical nociception in the rat. Neurosci Lett. 1989 Feb 13;97(1-2):198–202. doi: 10.1016/0304-3940(89)90163-8. [DOI] [PubMed] [Google Scholar]
- Hargreaves K. M., Troullos E. S., Dionne R. A., Schmidt E. A., Schafer S. C., Joris J. L. Bradykinin is increased during acute and chronic inflammation: therapeutic implications. Clin Pharmacol Ther. 1988 Dec;44(6):613–621. doi: 10.1038/clpt.1988.202. [DOI] [PubMed] [Google Scholar]
- Hock F. J., Wirth K., Albus U., Linz W., Gerhards H. J., Wiemer G., Henke S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br J Pharmacol. 1991 Mar;102(3):769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang E., Novak A., Reeh P. W., Handwerker H. O. Chemosensitivity of fine afferents from rat skin in vitro. J Neurophysiol. 1990 Apr;63(4):887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- McGuirk S. M., Vallis Y., Pasternak C. A., Dolphin A. C. Bradykinin enhances excitability in cultured rat sensory neurones by a GTP-dependent mechanism. Neurosci Lett. 1989 Apr 24;99(1-2):85–89. doi: 10.1016/0304-3940(89)90269-3. [DOI] [PubMed] [Google Scholar]
- Mense S., Schmidt R. F. Activation of group IV afferent units from muscle by algesic agents. Brain Res. 1974 Jun 7;72(2):305–310. doi: 10.1016/0006-8993(74)90870-1. [DOI] [PubMed] [Google Scholar]
- Rane S. G., Walsh M. P., McDonald J. R., Dunlap K. Specific inhibitors of protein kinase C block transmitter-induced modulation of sensory neuron calcium current. Neuron. 1989 Aug;3(2):239–245. doi: 10.1016/0896-6273(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. Depolarization of nonmyelinated fibers of the rat vagus nerve produced by activation of protein kinase C. J Neurosci. 1988 Jul;8(7):2606–2617. doi: 10.1523/JNEUROSCI.08-07-02606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Reynolds M. L., Fitzgerald M., Benowitz L. I. GAP-43 expression in developing cutaneous and muscle nerves in the rat hindlimb. Neuroscience. 1991;41(1):201–211. doi: 10.1016/0306-4522(91)90210-f. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., Farmer S. G., Burch R. M. Antagonists of B2 bradykinin receptors. FASEB J. 1989 Jul;3(9):2019–2025. doi: 10.1096/fasebj.3.9.2545496. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., Manning D. C., DeHaas C. J., Ferkany J. W., Borosky S. A., Connor J. R., Vavrek R. J., Stewart J. M., Snyder S. H. Bradykinin as a pain mediator: receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc Natl Acad Sci U S A. 1988 May;85(9):3245–3249. doi: 10.1073/pnas.85.9.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo Y. O., Levine J. D. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44(1):131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavrek R. J., Stewart J. M. Competitive antagonists of bradykinin. Peptides. 1985 Mar-Apr;6(2):161–164. doi: 10.1016/0196-9781(85)90033-6. [DOI] [PubMed] [Google Scholar]
- Weinreich D. Bradykinin inhibits a slow spike afterhyperpolarization in visceral sensory neurons. Eur J Pharmacol. 1986 Dec 2;132(1):61–63. doi: 10.1016/0014-2999(86)90010-5. [DOI] [PubMed] [Google Scholar]
- Weinreich D., Wonderlin W. F. Inhibition of calcium-dependent spike after-hyperpolarization increases excitability of rabbit visceral sensory neurones. J Physiol. 1987 Dec;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]


