Abstract
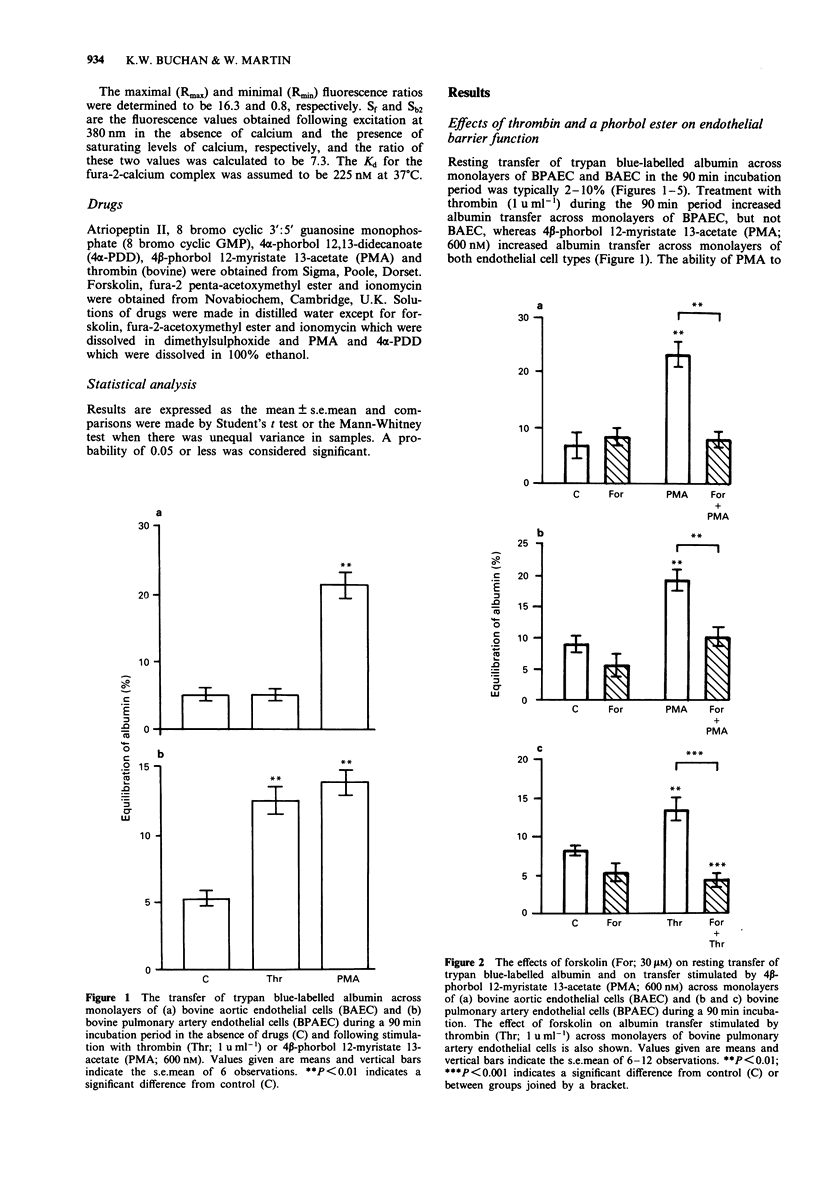
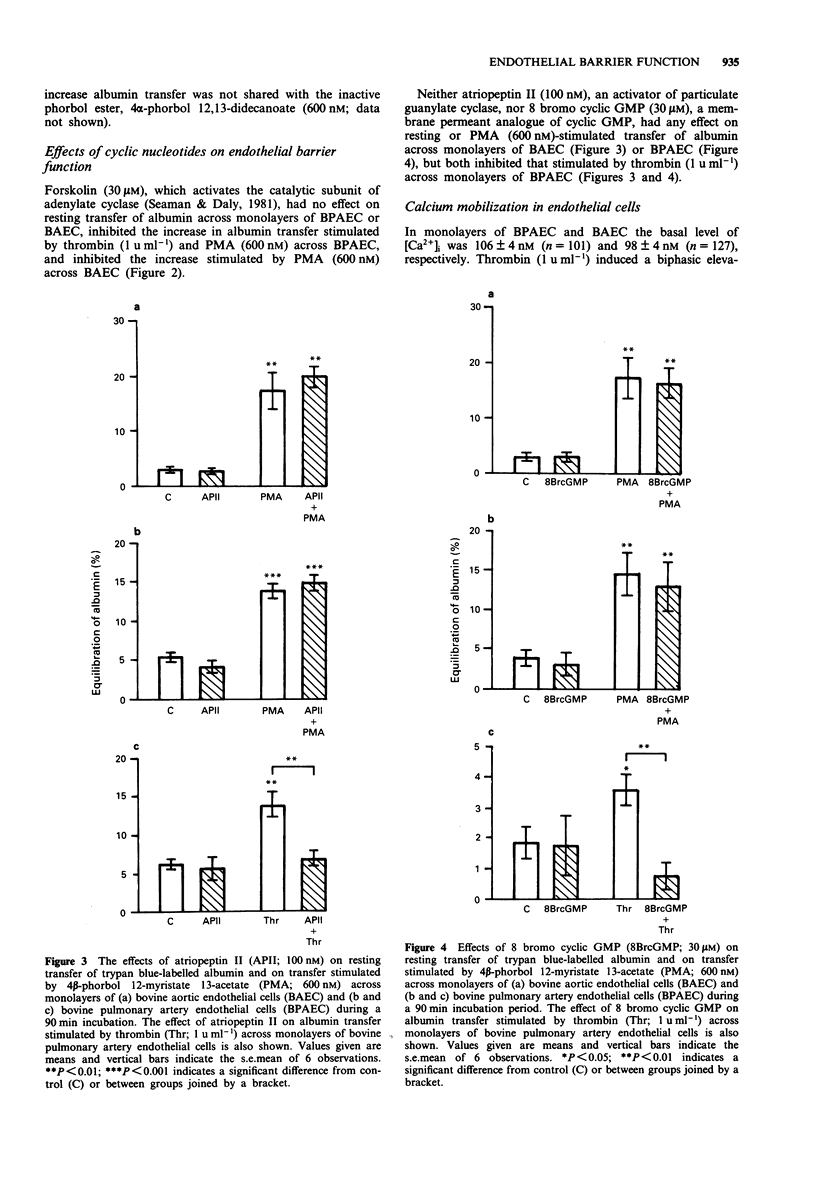
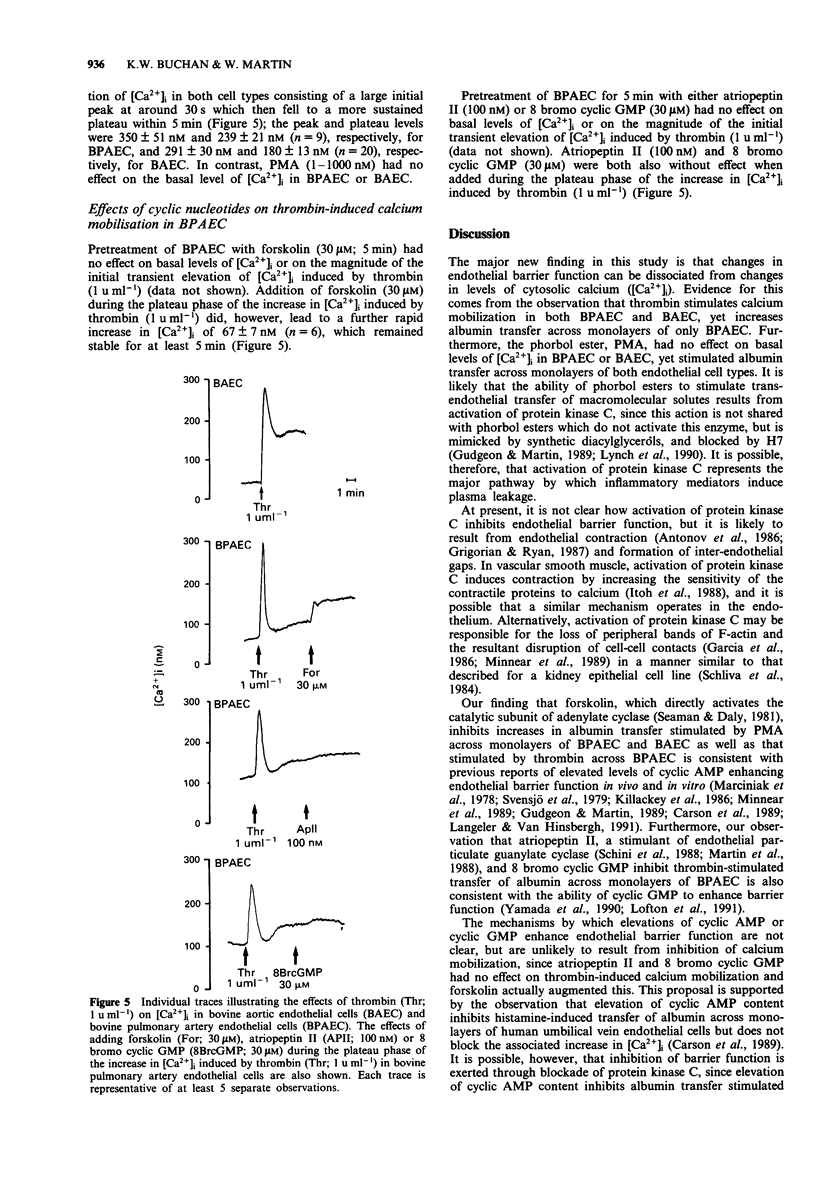
1. Barrier function and cytosolic free calcium content [Ca2+]i was measured in monolayers of bovine pulmonary artery endothelial cells (BPAEC) and bovine aortic endothelial cells (BAEC). 2. Thrombin (1 u ml-1) increased albumin transfer across monolayers of BPAEC but not BAEC, yet induced biphasic increases in [Ca2+]i in both endothelial cell types, consisting of a rapid, initial phasic component which decayed to a lower, more sustained plateau phase. 3. 4 beta-Phorbol 12-myristate 13-acetate (PMA; 0.3-3000 nM) increased albumin transfer across monolayers of BPAEC and BAEC, but had no effect on basal levels of [Ca2+]i in either endothelial cell type. 4. Treatment of BPAEC and BAEC with forskolin (30 microM), an activator of adenylate cyclase, had no effect on resting transfer of albumin, but inhibited that stimulated by PMA (600 nM). It also inhibited the thrombin (1 u ml-1)-induced increase in albumin transfer across monolayers of BPAEC, but enhanced the plateau phase of the associated increase in [Ca2+]i. 5. Treatment of BPAEC and BAEC with either atriopeptin II (100 nM), an activator of particulate guanylate cyclase, or 8 bromo cyclic GMP (30 microM) had no effect on resting or PMA (600 nM)-stimulated transfer of albumin. Both agents did, however, inhibit the thrombin (1 u ml-1)-induced increase in albumin transfer across monolayers of BPAEC, but had no effect on the associated increase in [Ca2+]i. 6. These data suggest a dissociation between the ability of agents that increase or decrease albumin transfer and their effects on [Ca2+]i.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonov A. S., Lukashev M. E., Romanov Y. A., Tkachuk V. A., Repin V. S., Smirnov V. N. Morphological alterations in endothelial cells from human aorta and umbilical vein induced by forskolin and phorbol 12-myristate 13-acetate: a synergistic action of adenylate cyclase and protein kinase C activators. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9704–9708. doi: 10.1073/pnas.83.24.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatch G. N., Abraham S., MacLeod B. A., Yoshida N. R., Walker M. J. Antiarrhythmic properties of tedisamil (KC8857), a putative transient outward K+ current blocker. Br J Pharmacol. 1991 Jan;102(1):13–18. doi: 10.1111/j.1476-5381.1991.tb12124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan K. W., Martin W. Bradykinin induces elevations of cytosolic calcium through mobilisation of intracellular and extracellular pools in bovine aortic endothelial cells. Br J Pharmacol. 1991 Jan;102(1):35–40. doi: 10.1111/j.1476-5381.1991.tb12128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M. R., Shasby S. S., Shasby D. M. Histamine and inositol phosphate accumulation in endothelium: cAMP and a G protein. Am J Physiol. 1989 Oct;257(4 Pt 1):L259–L264. doi: 10.1152/ajplung.1989.257.4.L259. [DOI] [PubMed] [Google Scholar]
- Casnocha S. A., Eskin S. G., Hall E. R., McIntire L. V. Permeability of human endothelial monolayers: effect of vasoactive agonists and cAMP. J Appl Physiol (1985) 1989 Nov;67(5):1997–2005. doi: 10.1152/jappl.1989.67.5.1997. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Haines K. A. Stimulus-response uncoupling in the neutrophil. Adenosine A2-receptor occupancy inhibits the sustained, but not the early, events of stimulus transduction in human neutrophils by a mechanism independent of actin-filament formation. Biochem J. 1992 Feb 1;281(Pt 3):631–635. doi: 10.1042/bj2810631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Rosenstein E. D., Korchak H. M., Weissmann G., Hirschhorn R. Occupancy of adenosine receptors raises cyclic AMP alone and in synergy with occupancy of chemoattractant receptors and inhibits membrane depolarization. Biochem J. 1988 Jun 15;252(3):709–715. doi: 10.1042/bj2520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. G., Siflinger-Birnboim A., Bizios R., Del Vecchio P. J., Fenton J. W., 2nd, Malik A. B. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986 Jul;128(1):96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- Grigorian G. Y., Ryan U. S. Platelet-activating factor effects on bovine pulmonary artery endothelial cells. Circ Res. 1987 Sep;61(3):389–395. doi: 10.1161/01.res.61.3.389. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gudgeon J. R., Martin W. Modulation of arterial endothelial permeability: studies on an in vitro model. Br J Pharmacol. 1989 Dec;98(4):1267–1274. doi: 10.1111/j.1476-5381.1989.tb12673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kubota Y., Kuriyama H. Effects of a phorbol ester on acetylcholine-induced Ca2+ mobilization and contraction in the porcine coronary artery. J Physiol. 1988 Mar;397:401–419. doi: 10.1113/jphysiol.1988.sp017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Killackey J. J., Johnston M. G., Movat H. Z. Increased permeability of microcarrier-cultured endothelial monolayers in response to histamine and thrombin. A model for the in vitro study of increased vasopermeability. Am J Pathol. 1986 Jan;122(1):50–61. [PMC free article] [PubMed] [Google Scholar]
- Lang D., Lewis M. J. Inhibition of inositol 1,4,5-trisphosphate formation by cyclic GMP in cultured aortic endothelial cells of the pig. Br J Pharmacol. 1991 Jan;102(1):277–281. doi: 10.1111/j.1476-5381.1991.tb12166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeler E. G., van Hinsbergh V. W. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol. 1991 May;260(5 Pt 1):C1052–C1059. doi: 10.1152/ajpcell.1991.260.5.C1052. [DOI] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Johns A., Rubanyi G. M., van Breemen C. Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990 Feb 15;265(5):2613–2619. [PubMed] [Google Scholar]
- Lofton C. E., Baron D. A., Heffner J. E., Currie M. G., Newman W. H. Atrial natriuretic peptide inhibits oxidant-induced increases in endothelial permeability. J Mol Cell Cardiol. 1991 Aug;23(8):919–927. doi: 10.1016/0022-2828(91)90134-8. [DOI] [PubMed] [Google Scholar]
- Lum H., Del Vecchio P. J., Schneider A. S., Goligorsky M. S., Malik A. B. Calcium dependence of the thrombin-induced increase in endothelial albumin permeability. J Appl Physiol (1985) 1989 Mar;66(3):1471–1476. doi: 10.1152/jappl.1989.66.3.1471. [DOI] [PubMed] [Google Scholar]
- Lynch J. J., Ferro T. J., Blumenstock F. A., Brockenauer A. M., Malik A. B. Increased endothelial albumin permeability mediated by protein kinase C activation. J Clin Invest. 1990 Jun;85(6):1991–1998. doi: 10.1172/JCI114663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak D. L., Dobbins D. E., Maciejko J. J., Scott J. B., Haddy F. J., Grega G. J. Antagonism of histamine edema formation by catecholamines. Am J Physiol. 1978 Feb;234(2):H180–H185. doi: 10.1152/ajpheart.1978.234.2.H180. [DOI] [PubMed] [Google Scholar]
- Martin W., White D. G., Henderson A. H. Endothelium-derived relaxing factor and atriopeptin II elevate cyclic GMP levels in pig aortic endothelial cells. Br J Pharmacol. 1988 Jan;93(1):229–239. doi: 10.1111/j.1476-5381.1988.tb11426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnear F. L., DeMichele M. A., Moon D. G., Rieder C. L., Fenton J. W., 2nd Isoproterenol reduces thrombin-induced pulmonary endothelial permeability in vitro. Am J Physiol. 1989 Nov;257(5 Pt 2):H1613–H1623. doi: 10.1152/ajpheart.1989.257.5.H1613. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schini V., Grant N. J., Miller R. C., Takeda K. Morphological characterization of cultured bovine aortic endothelial cells and the effects of atriopeptin II and sodium nitroprusside on cellular and extracellular accumulation of cyclic GMP. Eur J Cell Biol. 1988 Oct;47(1):53–61. [PubMed] [Google Scholar]
- Schliwa M., Nakamura T., Porter K. R., Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984 Sep;99(3):1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Stelzner T. J., Weil J. V., O'Brien R. F. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J Cell Physiol. 1989 Apr;139(1):157–166. doi: 10.1002/jcp.1041390122. [DOI] [PubMed] [Google Scholar]
- Svensjö E., Arfors K. E., Raymond R. M., Grega G. J. Morphological and physiological correlation of bradykinin-induced macromolecular efflux. Am J Physiol. 1979 Apr;236(4):H600–H606. doi: 10.1152/ajpheart.1979.236.4.H600. [DOI] [PubMed] [Google Scholar]
- Thompson N. T., Bonser R. W., Garland L. G. Receptor-coupled phospholipase D and its inhibition. Trends Pharmacol Sci. 1991 Nov;12(11):404–408. doi: 10.1016/0165-6147(91)90617-2. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Furumichi T., Furui H., Yokoi T., Ito T., Yamauchi K., Yokota M., Hayashi H., Saito H. Roles of calcium, cyclic nucleotides, and protein kinase C in regulation of endothelial permeability. Arteriosclerosis. 1990 May-Jun;10(3):410–420. doi: 10.1161/01.atv.10.3.410. [DOI] [PubMed] [Google Scholar]


