Abstract
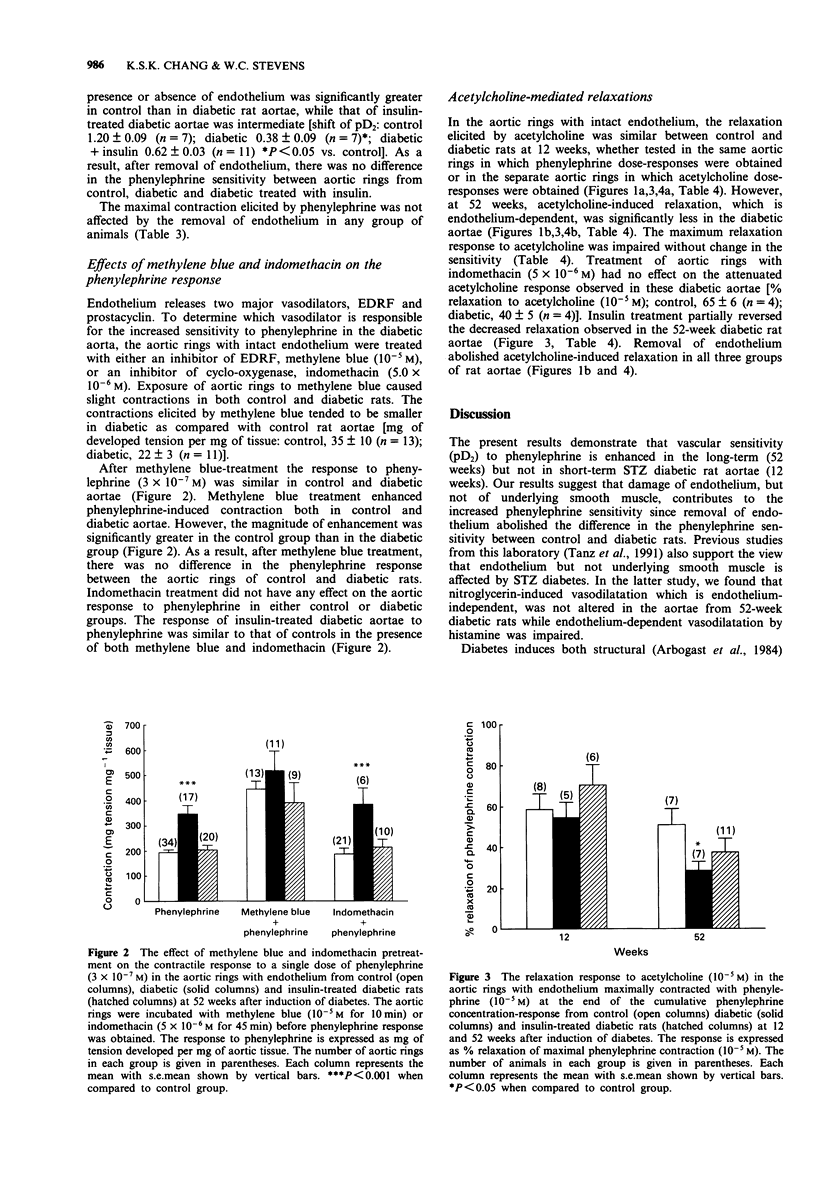
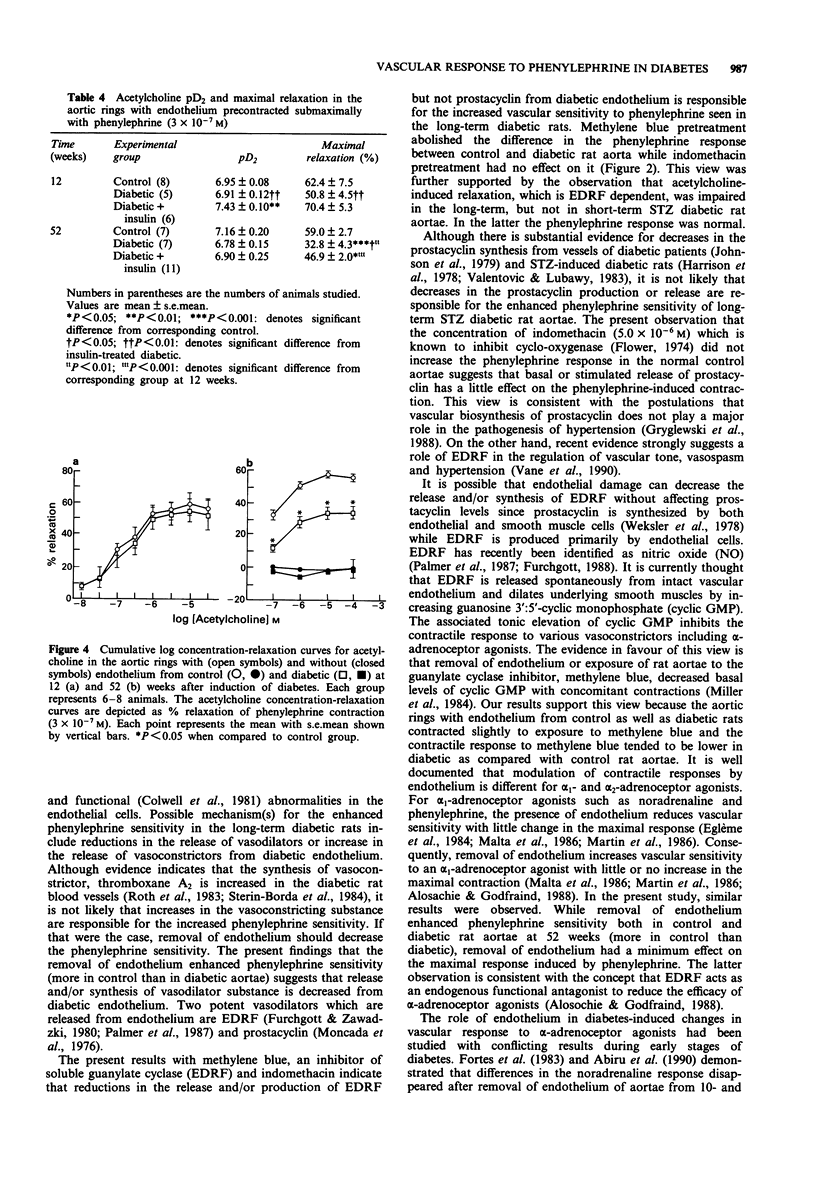
1. The effect of short- and long-term streptozotocin (STZ)-induced diabetes (12 and 52 weeks) on the vascular response to phenylephrine was examined in the isolated thoracic aorta with and without intact endothelium from diabetic, age matched control rats and diabetic rats treated with insulin. 2. Twelve weeks after induction of diabetes, aortae with intact endothelium demonstrated no changes either in sensitivity (defined as pD2) or contractility (defined as the maximal developed tension per aortic tissue wet weight) to phenylephrine. 3. In contrast, 52 weeks after induction of diabetes, aortae with intact endothelium demonstrated an increased sensitivity to phenylephrine while contractility to phenylephrine was not changed. Insulin treatment partially corrected the increased sensitivity to phenylephrine observed in diabetic rat aorta. 4. Removal of endothelium abolished the difference in phenylephrine sensitivity between diabetic and control aortae at 52 weeks. 5. Pretreatment of intact aortae with methylene blue, an inhibitor of endothelium-derived relaxing factor (EDRF), abolished the difference in phenylephrine sensitivity between control and diabetic rat aortae at 52 weeks, while pretreatment with indomethacin, an inhibitor of cyclo-oxygenase, had no effect. These results suggest that decreases in production or release of EDRF might be responsible for the increased vascular sensitivity to phenylephrine observed in long-term STZ diabetic rats. 6. Acetylcholine-induced relaxation, which is EDRF-dependent, was less in diabetic rat aortae with intact endothelium at 52 weeks, but not at 12 weeks. These results further support the theory that decreases in capacity of the endothelium to synthesize or release EDRF may occur in long-term STZ diabetic rats.
Full text
PDF

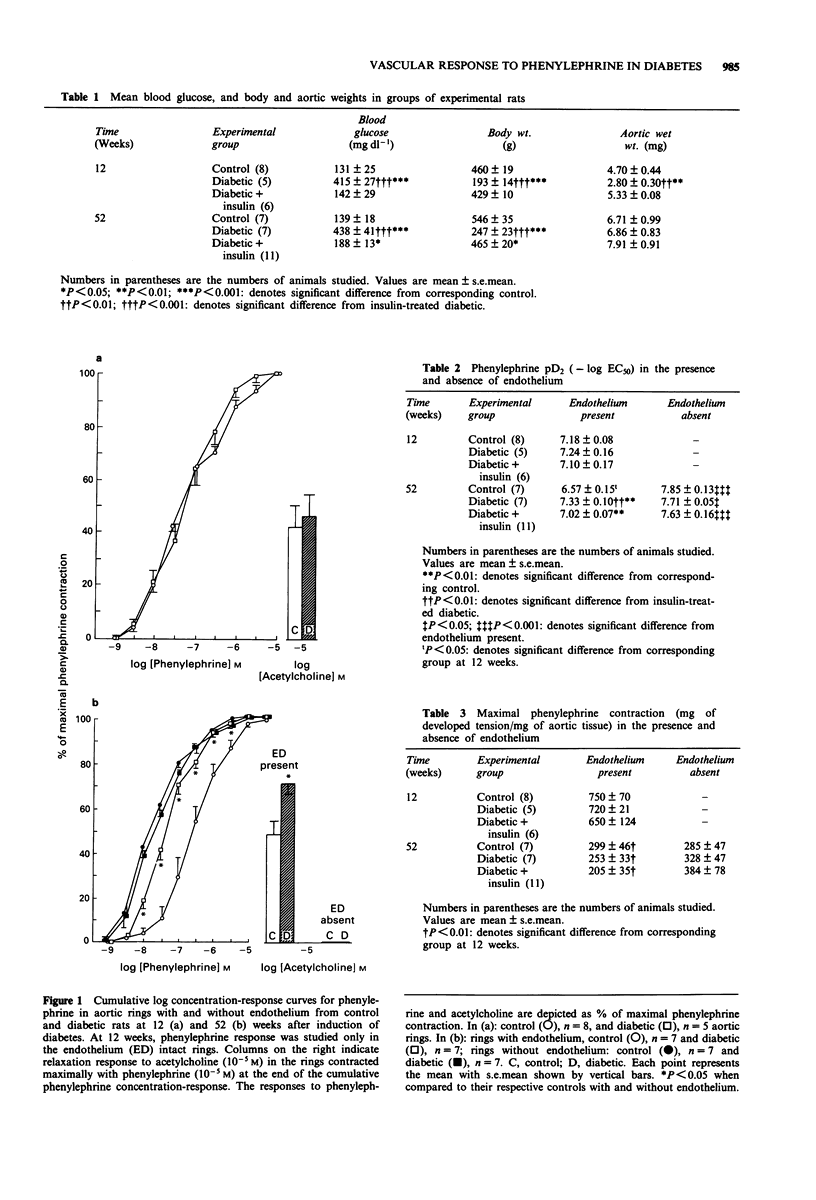



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiru T., Kamata K., Miyata N., Kasuya Y. Differences in vascular responses to vasoactive agents of basilar artery and aorta from rabbits with alloxan-induced diabetes. Can J Physiol Pharmacol. 1990 Jul;68(7):882–888. doi: 10.1139/y90-134. [DOI] [PubMed] [Google Scholar]
- Agrawal D. K., McNeill J. H. Vascular responses to agonists in rat mesenteric artery from diabetic rats. Can J Physiol Pharmacol. 1987 Jul;65(7):1484–1490. doi: 10.1139/y87-232. [DOI] [PubMed] [Google Scholar]
- Alosachie I., Godfraind T. The modulatory role of vascular endothelium in the interaction of agonists and antagonists with alpha-adrenoceptors in the rat aorta. Br J Pharmacol. 1988 Oct;95(2):619–629. doi: 10.1111/j.1476-5381.1988.tb11684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast B. W., Berry D. L., Newell C. L. Injury of arterial endothelial cells in diabetic, sucrose-fed and aged rats. Atherosclerosis. 1984 Apr;51(1):31–45. doi: 10.1016/0021-9150(84)90142-4. [DOI] [PubMed] [Google Scholar]
- Carrier G. O., White R. E. Enhancement of alpha-1 and alpha-2 adrenergic agonist-induced vasoconstriction by removal of endothelium in rat aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):682–687. [PubMed] [Google Scholar]
- Chang K. S., Lacy M. O., Stevens W. C. Heart rate and blood pressure response to methacholine in long term streptozotocin diabetic rats. Proc West Pharmacol Soc. 1988;31:173–176. [PubMed] [Google Scholar]
- Christlieb A. R. Diabetes and hypertensive vascular disease. Mechanisms and treatment. Am J Cardiol. 1973 Sep 20;32(4):592–606. doi: 10.1016/s0002-9149(73)80051-7. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983 Oct 13;305(5935):627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- Colwell J. A., Halushka P. V., Sarji K. E., Lopes-Virella M. F., Sagel J. Vascular disease in diabetes: pathophysiological mechanisms and therapy. Arch Intern Med. 1979 Feb;139(2):225–230. [PubMed] [Google Scholar]
- Dolgov V. V., Zaikina O. E., Bondarenko M. F., Repin V. S. Aortic endothelium of alloxan diabetic rabbits: a quantitative study using scanning electron microscopy. Diabetologia. 1982 May;22(5):338–343. doi: 10.1007/BF00253578. [DOI] [PubMed] [Google Scholar]
- Durante W., Sen A. K., Sunahara F. A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988 Jun;94(2):463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglème C., Godfraind T., Miller R. C. Enhanced responsiveness of rat isolated aorta to clonidine after removal of the endothelial cells. Br J Pharmacol. 1984 Jan;81(1):16–18. doi: 10.1111/j.1476-5381.1984.tb10736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming W. W., Westfall D. P., De la Lande I. S., Jellett L. B. Log-normal distribution of equiefective doses of norepinephrine and acetylcholine in several tissues. J Pharmacol Exp Ther. 1972 May;181(2):339–345. [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Fortes Z. B., Garcia Leme J., Scivoletto R. Vascular reactivity in diabetes mellitus: role of the endothelial cell. Br J Pharmacol. 1983 Jul;79(3):771–781. doi: 10.1111/j.1476-5381.1983.tb10016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Inhibition of rat mesangial cell mitogenesis by nitric oxide-generating vasodilators. Am J Physiol. 1989 Jul;257(1 Pt 2):F60–F66. doi: 10.1152/ajprenal.1989.257.1.F60. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D., Koltai M. Z., Pogátsa G., Magyar K., Hadházy P. Altered responsiveness of diabetic dog renal arteries to acetylcholine and phenylephrine: role of endothelium. Pharmacology. 1989;38(3):177–184. doi: 10.1159/000138535. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D., Koltai M. Z., Pogátsa G., Magyar K., Hadházy P. Differential contractile responsiveness of femoral arteries from healthy and diabetic dogs: role of endothelium. Arch Int Pharmacodyn Ther. 1987 Jul;288(1):100–108. [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Botting R. M., Vane J. R. Mediators produced by the endothelial cell. Hypertension. 1988 Dec;12(6):530–548. doi: 10.1161/01.hyp.12.6.530. [DOI] [PubMed] [Google Scholar]
- Harris K. H., MacLeod K. M. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur J Pharmacol. 1988 Aug 9;153(1):55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- Harrison H. E., Reece A. H., Johnson M. Decreased vascular prostacyclin in experimental diabetes. Life Sci. 1978 Jul 24;23(4):351–355. doi: 10.1016/0024-3205(78)90020-6. [DOI] [PubMed] [Google Scholar]
- Head R. J., Longhurst P. A., Panek R. L., Stitzel R. E. A contrasting effect of the diabetic state upon the contractile responses of aortic preparations from the rat and rabbit. Br J Pharmacol. 1987 Jun;91(2):275–286. doi: 10.1111/j.1476-5381.1987.tb10282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. V., Carrier G. O. Supersensitivity of isolated mesenteric arteries to noradrenaline in the long-term experimental diabetic rat. J Auton Pharmacol. 1981 Dec;1(5):399–405. doi: 10.1111/j.1474-8673.1981.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Johnson M., Harrison H. E., Raftery A. T., Elder J. B. Vascular prostacyclin may be reduced in diabetes in man. Lancet. 1979 Feb 10;1(8111):325–326. doi: 10.1016/s0140-6736(79)90737-2. [DOI] [PubMed] [Google Scholar]
- MacLeod K. M., McNeill J. H. The influence of chronic experimental diabetes on contractile responses of rat isolated blood vessels. Can J Physiol Pharmacol. 1985 Jan;63(1):52–57. doi: 10.1139/y85-009. [DOI] [PubMed] [Google Scholar]
- Malta E., Schini V., Miller R. C. Role of efficacy in the assessment of the actions of alpha-adrenoceptor agonists in rat aorta with endothelium. J Pharm Pharmacol. 1986 Mar;38(3):209–213. doi: 10.1111/j.2042-7158.1986.tb04545.x. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Meraji S., Jayakody L., Senaratne M. P., Thomson A. B., Kappagoda T. Endothelium-dependent relaxation in aorta of BB rat. Diabetes. 1987 Aug;36(8):978–981. doi: 10.2337/diab.36.8.978. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Mony M., Schini V., Schoeffter P., Stoclet J. C. Endothelial mediated inhibition of contraction and increase in cyclic GMP levels evoked by the alpha-adrenoceptor agonist B-HT 920 in rat isolated aorta. Br J Pharmacol. 1984 Dec;83(4):903–908. doi: 10.1111/j.1476-5381.1984.tb16530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pfaffman M. A., Ball C. R., Darby A., Hilman R. Insulin reversal of diabetes-induced inhibition of vascular contractility in the rat. Am J Physiol. 1982 Apr;242(4):H490–H495. doi: 10.1152/ajpheart.1982.242.4.H490. [DOI] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Roth D. M., Reibel D. K., Lefer A. M. Vascular responsiveness and eicosanoid production in diabetic rats. Diabetologia. 1983 May;24(5):372–376. doi: 10.1007/BF00251827. [DOI] [PubMed] [Google Scholar]
- Scarborough N. L., Carrier G. O. Nifedipine and alpha adrenoceptors in rat aorta. II. Role of extracellular calcium in enhanced alpha-2 adrenoceptor-mediated contraction in diabetes. J Pharmacol Exp Ther. 1984 Dec;231(3):603–609. [PubMed] [Google Scholar]
- Sterin-Borda L., Franchi A. M., Borda E. S., Del Castillo E., Gimeno M. F., Gimeno A. L. Augmented thromboxane generation by mesenteric arteries from pancreatectomized diabetic dogs is coincident with the vascular tone enhancement evoked by Na arachidonate and prostacyclin. Eur J Pharmacol. 1984 Aug 17;103(3-4):211–221. doi: 10.1016/0014-2999(84)90480-1. [DOI] [PubMed] [Google Scholar]
- Valentovic M. A., Lubawy W. C. Impact of insulin or tolbutamide treatment on 14C-arachidonic acid conversion to prostacyclin and/or thromboxane in lungs, aortas, and platelets of streptozotocin-induced diabetic rats. Diabetes. 1983 Sep;32(9):846–851. doi: 10.2337/diab.32.9.846. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I., Hatake K., Kimura N., Kakishita E., Nagai K. Modulation of vascular tonus by the endothelium in experimental diabetes. Life Sci. 1987 Feb 16;40(7):643–648. doi: 10.1016/0024-3205(87)90265-7. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., Carrier G. O. Enhanced vascular alpha-adrenergic neuroeffector system in diabetes: importance of calcium. Am J Physiol. 1988 Nov;255(5 Pt 2):H1036–H1042. doi: 10.1152/ajpheart.1988.255.5.H1036. [DOI] [PubMed] [Google Scholar]


