Abstract
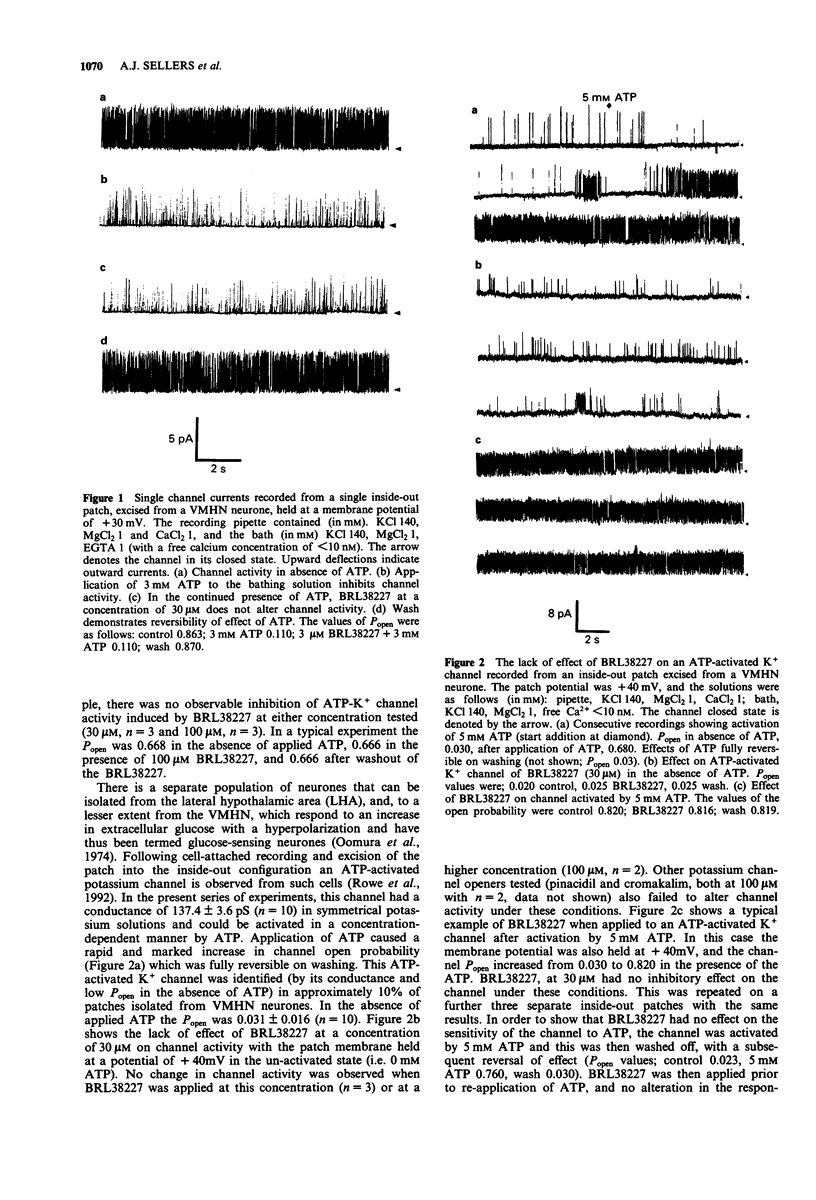
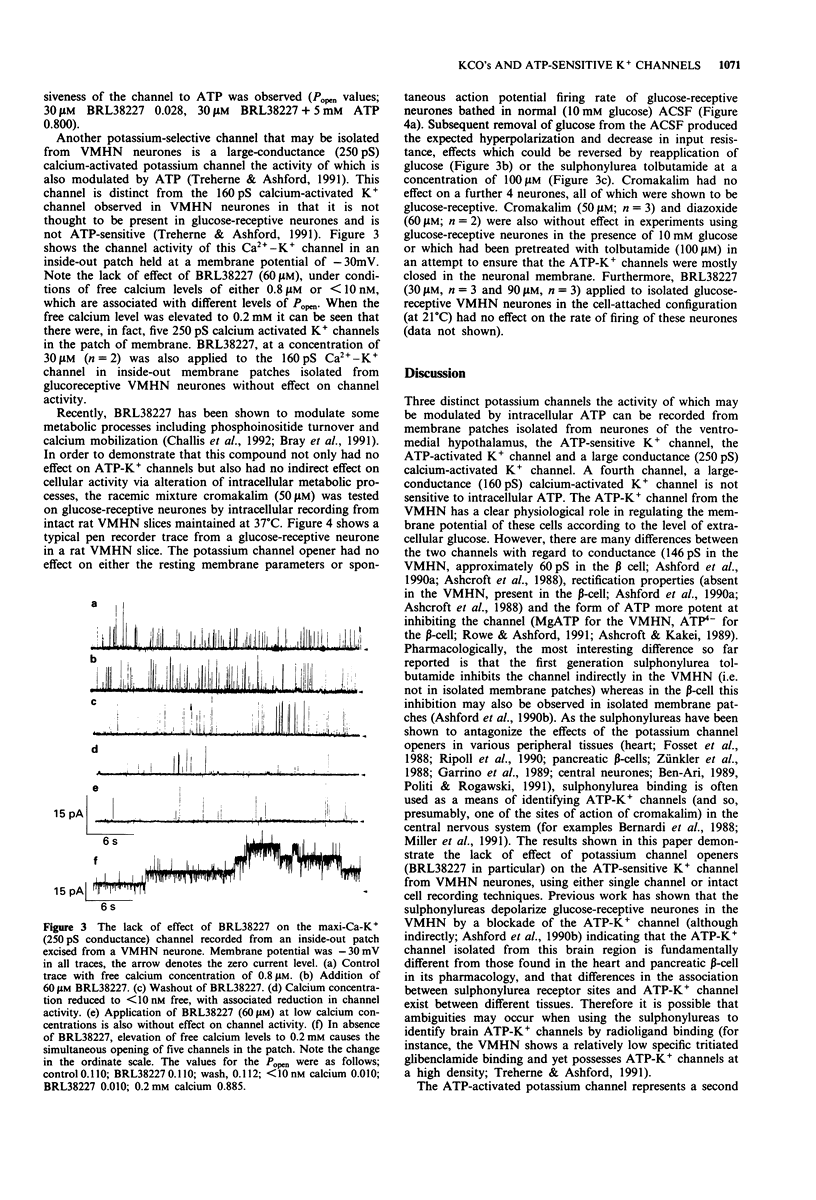
1. Single neuronal cells were freshly isolated from the ventromedial hypothalamic nuclei (VMHN) of the rat brain. Currents through ATP-modulated and large conductance (160 and 250 pS) calcium-activated potassium channels were recorded by the cell-attached and excised inside-out patch techniques. 2. BRL38227 (lemakalim; 30-90 microM) applied to the superfusing medium produced no change in firing rate of isolated glucose-receptive VMHN neurones in cell-attached recordings. 3. BRL38227, at concentrations of between 30-100 microM applied to the intracellular (cytoplasmic) aspect of inside-out patches, had no effect on the activity of ATP-sensitive K+ channels in the absence of ATP or in the presence of a sub-maximal inhibitory concentration (3 mM) of ATP. Cromakalim, pinacidil, minoxidil sulphate and diazoxide also produced no effect under these conditions. 4. The potassium channel openers (KCO's) were tested on ATP-activated potassium channels recorded from a further subpopulation of VMHN neurones. Application of BRL38227 (up to and including 100 microM) to this channel in inside-out patches either in the absence of ATP or when activated by 5 mM ATP had no effect on channel activity. Identical results were obtained with cromakalim and pinacidil. 5. BRL38227 had no effect on either of the large conductance (250 pS and 160 pS) calcium-activated potassium channels in VMHN neurones. 6. Intracellular recordings were made from glucose-receptive VMHN neurones in rat brain slices. Cromakalim (50 microM) or diazoxide (60 microM) did not alter the firing rate or passive membrane properties of these neurones demonstrated to be sensitive to tolbutamide (0.1 mM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzheimer C., ten Bruggencate G. Actions of BRL 34915 (Cromakalim) upon convulsive discharges in guinea pig hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol. 1988 Apr;337(4):429–434. doi: 10.1007/BF00169535. [DOI] [PubMed] [Google Scholar]
- Aronson J. K. Potassium channels in nervous tissue. Biochem Pharmacol. 1992 Jan 9;43(1):11–14. doi: 10.1016/0006-2952(92)90653-z. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Properties of single potassium channels modulated by glucose in rat pancreatic beta-cells. J Physiol. 1988 Jun;400:501–527. doi: 10.1113/jphysiol.1988.sp017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Kakei M. ATP-sensitive K+ channels in rat pancreatic beta-cells: modulation by ATP and Mg2+ ions. J Physiol. 1989 Sep;416:349–367. doi: 10.1113/jphysiol.1989.sp017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990 Jan;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurones by indirect inhibition of ATP-K+ channels. Br J Pharmacol. 1990 Nov;101(3):531–540. doi: 10.1111/j.1476-5381.1990.tb14116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi H., Fosset M., Lazdunski M. Characterization, purification, and affinity labeling of the brain [3H]glibenclamide-binding protein, a putative neuronal ATP-regulated K+ channel. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9816–9820. doi: 10.1073/pnas.85.24.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden P., Hill R. G. Effects of cholecystokinin and related peptides on neuronal activity in the ventromedial nucleus of the rat hypothalamus. Br J Pharmacol. 1988 May;94(1):246–252. doi: 10.1111/j.1476-5381.1988.tb11521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray K. M., Weston A. H., Duty S., Newgreen D. T., Longmore J., Edwards G., Brown T. J. Differences between the effects of cromakalim and nifedipine on agonist-induced responses in rabbit aorta. Br J Pharmacol. 1991 Feb;102(2):337–344. doi: 10.1111/j.1476-5381.1991.tb12175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Patel N., Arch J. R. Comparative effects of BRL 38227, nitrendipine and isoprenaline on carbachol- and histamine-stimulated phosphoinositide metabolism in airway smooth muscle. Br J Pharmacol. 1992 Apr;105(4):997–1003. doi: 10.1111/j.1476-5381.1992.tb09091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande D., Thuringer D., Leguern S., Cavero I. The potassium channel opener cromakalim (BRL 34915) activates ATP-dependent K+ channels in isolated cardiac myocytes. Biochem Biophys Res Commun. 1988 Jul 29;154(2):620–625. doi: 10.1016/0006-291x(88)90184-2. [DOI] [PubMed] [Google Scholar]
- Fan Z., Nakayama K., Hiraoka M. Pinacidil activates the ATP-sensitive K+ channel in inside-out and cell-attached patch membranes of guinea-pig ventricular myocytes. Pflugers Arch. 1990 Jan;415(4):387–394. doi: 10.1007/BF00373613. [DOI] [PubMed] [Google Scholar]
- Fosset M., De Weille J. R., Green R. D., Schmid-Antomarchi H., Lazdunski M. Antidiabetic sulfonylureas control action potential properties in heart cells via high affinity receptors that are linked to ATP-dependent K+ channels. J Biol Chem. 1988 Jun 15;263(17):7933–7936. [PubMed] [Google Scholar]
- Gandolfo G., Gottesmann C., Bidard J. N., Lazdunski M. K+ channels openers prevent epilepsy induced by the bee venom peptide MCD. Eur J Pharmacol. 1989 Jan 17;159(3):329–330. doi: 10.1016/0014-2999(89)90169-6. [DOI] [PubMed] [Google Scholar]
- Gandolfo G., Romettino S., Gottesmann C., van Luijtelaar G., Coenen A., Bidard J. N., Lazdunski M. K+ channel openers decrease seizures in genetically epileptic rats. Eur J Pharmacol. 1989 Aug 11;167(1):181–183. doi: 10.1016/0014-2999(89)90762-0. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Plant T. D., Henquin J. C. Effects of putative activators of K+ channels in mouse pancreatic beta-cells. Br J Pharmacol. 1989 Nov;98(3):957–965. doi: 10.1111/j.1476-5381.1989.tb14626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband C. H., Silberberg S. D., Groschner K., van Breemen C. ATP inhibits smooth muscle Ca2(+)-activated K+ channels. Proc Biol Sci. 1990 Oct 22;242(1303):23–28. doi: 10.1098/rspb.1990.0098. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hunter M., Giebisch G. Calcium-activated K-channels of Amphiuma early distal tubule: inhibition by ATP. Pflugers Arch. 1988 Aug;412(3):331–333. doi: 10.1007/BF00582517. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. ATP suppresses activity of Ca(2+)-activated K+ channels by Ca2+ chelation. Pflugers Arch. 1992 Jan;420(1):101–105. doi: 10.1007/BF00378648. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Hales C. N., Ashford M. L. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. Br J Pharmacol. 1989 Aug;97(4):1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K., Pavenstädt H., Greger R. Characterization of potassium channels in respiratory cells. II. Inhibitors and regulation. Pflugers Arch. 1989 Jul;414(3):297–303. doi: 10.1007/BF00584630. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Velayo N. L., Dage R. C., Rampe D. High affinity [3H]glibenclamide binding sites in rat neuronal and cardiac tissue: localization and developmental characteristics. J Pharmacol Exp Ther. 1991 Jan;256(1):358–364. [PubMed] [Google Scholar]
- Miller R. J. Glucose-regulated potassium channels are sweet news for neurobiologists. Trends Neurosci. 1990 Jun;13(6):197–199. doi: 10.1016/0166-2236(90)90158-7. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Ooyama H., Sugimori M., Nakamura T., Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974 Feb 1;247(5439):284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- Politi D. M., Rogawski M. A. Glyburide-sensitive K+ channels in cultured rat hippocampal neurons: activation by cromakalim and energy-depleting conditions. Mol Pharmacol. 1991 Aug;40(2):308–315. [PubMed] [Google Scholar]
- Quasthoff S., Spuler A., Lehmann-Horn F., Grafe P. Cromakalim, pinacidil and RP 49356 activate a tolbutamide-sensitive K+ conductance in human skeletal muscle fibres. Pflugers Arch. 1989;414 (Suppl 1):S179–S180. doi: 10.1007/BF00582294. [DOI] [PubMed] [Google Scholar]
- Ripoll C., Lederer W. J., Nichols C. G. Modulation of ATP-sensitive K+ channel activity and contractile behavior in mammalian ventricle by the potassium channel openers cromakalim and RP49356. J Pharmacol Exp Ther. 1990 Nov;255(2):429–435. [PubMed] [Google Scholar]
- Robertson D. W., Steinberg M. I. Potassium channel modulators: scientific applications and therapeutic promise. J Med Chem. 1990 Jun;33(6):1529–1541. doi: 10.1021/jm00168a001. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Scott A. L., Zingaro G. J., Siegl P. K. BRL 34915 (cromakalim) activates ATP-sensitive K+ current in cardiac muscle. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8360–8364. doi: 10.1073/pnas.85.21.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., Amoroso S., Fosset M., Lazdunski M. K+ channel openers activate brain sulfonylurea-sensitive K+ channels and block neurosecretion. Proc Natl Acad Sci U S A. 1990 May;87(9):3489–3492. doi: 10.1073/pnas.87.9.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess N. C., Kozlowski R. Z., Carrington C. A., Hales C. N., Ashford M. L. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol. 1988 Sep;95(1):83–94. doi: 10.1111/j.1476-5381.1988.tb16551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricklebank M. D., Flockhart G., Freedman S. B. The potassium channel activator, BRL 34915, antagonises a behavioural response to the muscarinic receptor agonist, pilocarpine. Eur J Pharmacol. 1988 Jul 7;151(2):349–350. doi: 10.1016/0014-2999(88)90824-2. [DOI] [PubMed] [Google Scholar]
- Weston A. H. Smooth muscle K+ channel openers; their pharmacology and clinical potential. Pflugers Arch. 1989;414 (Suppl 1):S99–105. doi: 10.1007/BF00582256. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Lenzen S., Männer K., Panten U., Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):225–230. doi: 10.1007/BF00169252. [DOI] [PubMed] [Google Scholar]


