Abstract
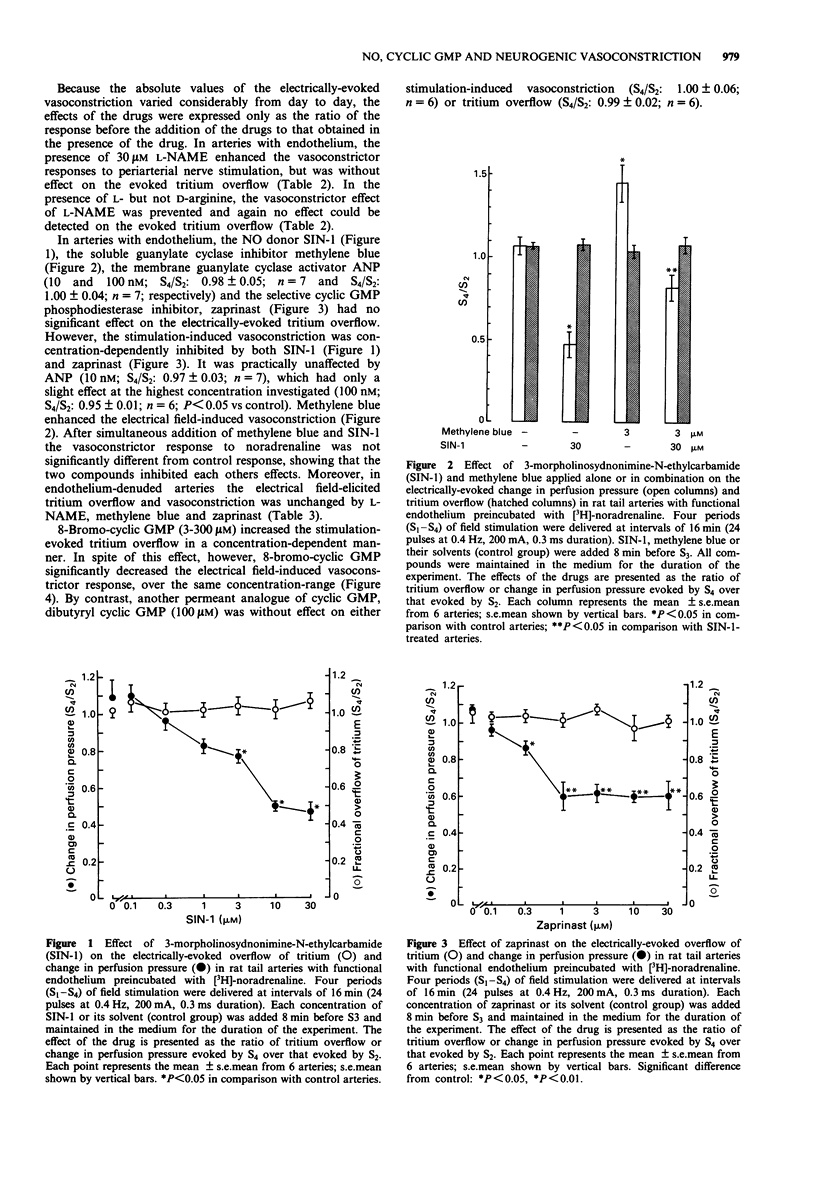
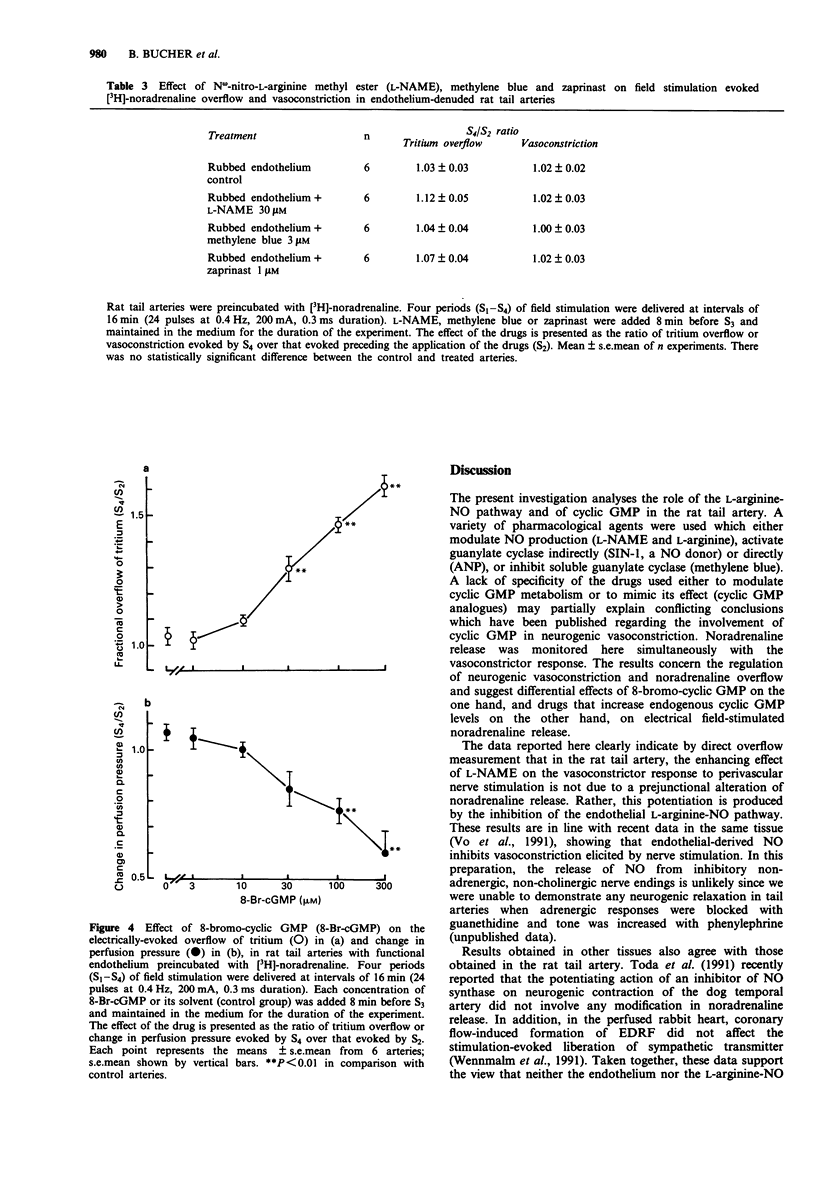
1. The possible roles of the L-arginine-NO pathway and of guanosine 3':5'-cyclic monophosphate (cyclic GMP) in regulating the prejunctional release of noradrenaline and neurogenic vasoconstriction were investigated in the perfused rat tail artery. 2. In the presence of N omega-nitro-L-arginine methyl ester (L-NAME; 30 microM), an inhibitor of NO formation, the vasoconstrictor responses to perivascular nerve stimulation (24 pulses at 0.4 Hz, 0.3 ms, 200 mA) and to exogenous noradrenaline (1 microM) were significantly enhanced, whereas the stimulation-evoked tritium overflow from [3H]-noradrenaline preloaded arteries was not modified. The vasoconstriction enhancing effect of L-NAME was prevented by L-arginine (1 mM) but not D-arginine (1 mM) and was abolished by removal of the endothelium. 3. The NO donor, 3-morpholinosydnonimine-N-ethylcarbamide (SIN-1; 0.1-30 microM), and the cyclic GMP phosphodiesterase inhibitor, zaprinast (0.1-30 microM) both induced a concentration-dependent inhibition of the electrical field stimulation-induced vasoconstriction, while atrial natriuretic peptide (ANP; 100 nM) produced only a slight decrease of the vasoconstrictor response. Methylene blue (3 microM), a known inhibitor of soluble guanylate cyclase increased the electrical field stimulation-induced vasoconstriction. SIN-1 and methylene blue when administered simultaneously, antagonized each others effect. None of the compounds tested (SIN-1, zaprinast, ANP or methylene blue) had any significant effect on the stimulation-evoked [3H]-noradrenaline overflow. 4. 8-Bromo-cyclic GMP, a potent activator of cyclic GMP-dependent protein kinase, markedly and concentration-dependently (3-300 microM) increased [3H]-noradrenaline overflow but decreased field stimulation-induced vasoconstriction.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlner J., Ljusegren M. E., Grundström N., Axelsson K. L. Role of nitric oxide and cyclic GMP as mediators of endothelium-independent neurogenic relaxation in bovine mesenteric artery. Circ Res. 1991 Mar;68(3):756–762. doi: 10.1161/01.res.68.3.756. [DOI] [PubMed] [Google Scholar]
- Axelsson K. L., Andersson R. G., Wikberg J. E. Effect of cGMP derivatives on contraction relaxation cycle, release of norepinephrine and protein kinase activity in guinea pig vas deferens. Acta Pharmacol Toxicol (Copenh) 1980 Nov;47(5):328–334. doi: 10.1111/j.1600-0773.1980.tb01568.x. [DOI] [PubMed] [Google Scholar]
- Bucher B., Bettermann R., Illes P. Plasma concentration and vascular effect of beta-endorphin in spontaneously hypertensive and Wistar Kyoto rats. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):428–432. doi: 10.1007/BF00165558. [DOI] [PubMed] [Google Scholar]
- Bucher B., Pain L., Stoclet J. C., Illes P. Role of cyclic AMP in the prejunctional alpha 2-adrenoceptor modulation of noradrenaline release from the rat tail artery. Naunyn Schmiedebergs Arch Pharmacol. 1990 Dec;342(6):640–649. doi: 10.1007/BF00175706. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Schenck K. W. Atriopeptin II: differential sensitivity of arteries and veins from the rat. Eur J Pharmacol. 1985 Jan 15;108(1):103–104. doi: 10.1016/0014-2999(85)90288-2. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Weisbrod R. M. Endothelium inhibits norepinephrine release from adrenergic nerves of rabbit carotid artery. Am J Physiol. 1988 May;254(5 Pt 2):H871–H878. doi: 10.1152/ajpheart.1988.254.5.H871. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Lincoln T. M. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem. 1989 Jan 15;264(2):1146–1155. [PubMed] [Google Scholar]
- Cubeddu L., 5th, Barnes E., Weiner N. Release of norepinephrine and dopamine-beta-hydroxylase by nerve stimulation. IV. An evaluation of a role for cyclic adenosine monophosphate. J Pharmacol Exp Ther. 1975 Apr;193(1):105–127. [PubMed] [Google Scholar]
- Faison E. P., Siegl P. K., Morgan G., Winquist R. J. Regional vasorelaxant selectivity of atrial natriuretic factor in isolated rabbit vessels. Life Sci. 1985 Sep 16;37(11):1073–1079. doi: 10.1016/0024-3205(85)90599-5. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Noblett B. D., Todd B. W., Wells J. N., Corbin J. D. Relaxation of vascular and tracheal smooth muscle by cyclic nucleotide analogs that preferentially activate purified cGMP-dependent protein kinase. Mol Pharmacol. 1988 Oct;34(4):506–517. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. S., Diecke F. P., Peevy K., Tanaka T. P. Release of norepinephrine from adrenergic nerve endings of blood vessels is modulated by endothelium-derived relaxing factor. Am J Hypertens. 1990 Mar;3(3):211–218. doi: 10.1093/ajh/3.3.211. [DOI] [PubMed] [Google Scholar]
- Greenberg S. S., Peevy K., Tanaka T. P. Endothelium-derived and intraneuronal nitric oxide-dependent inhibition of norepinephrine efflux from sympathetic nerves by bradykinin. Am J Hypertens. 1991 May;4(5 Pt 1):464–467. doi: 10.1093/ajh/4.5.464. [DOI] [PubMed] [Google Scholar]
- Greenberg S., Diecke F. P., Peevy K., Tanaka T. P. The endothelium modulates adrenergic neurotransmission to canine pulmonary arteries and veins. Eur J Pharmacol. 1989 Mar 14;162(1):67–80. doi: 10.1016/0014-2999(89)90605-5. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Kadowitz P. J., Ignarro L. J. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can J Physiol Pharmacol. 1981 Feb;59(2):150–156. doi: 10.1139/y81-025. [DOI] [PubMed] [Google Scholar]
- Halbrügge T., Lütsch K., Thyen A., Graefe K. H. Role of nitric oxide formation in the regulation of haemodynamics and the release of noradrenaline and adrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1991 Dec;344(6):720–727. doi: 10.1007/BF00174757. [DOI] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990 Nov;16(5):477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Illes P., Bettermann R., Brod I., Bucher B. Beta-endorphin-sensitive opioid receptors in the rat tail artery. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):420–427. doi: 10.1007/BF00165557. [DOI] [PubMed] [Google Scholar]
- Johnston H., Majewski H., Musgrave I. F. Involvement of cyclic nucleotides in prejunctional modulation of noradrenaline release in mouse atria. Br J Pharmacol. 1987 Aug;91(4):773–781. doi: 10.1111/j.1476-5381.1987.tb11275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S. Cyclic GMP as the mediator of molsidomine-induced vasodilatation. Eur J Pharmacol. 1986 Mar 11;122(1):103–109. doi: 10.1016/0014-2999(86)90164-0. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur J Pharmacol. 1990 Dec 4;191(3):303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Barbanti G., Turini D., Giuliani S. Effect of NG-monomethyl L-arginine (L-NMMA) and NG-nitro L-arginine (L-NOARG) on non-adrenergic non-cholinergic relaxation in the circular muscle of the human ileum. Br J Pharmacol. 1991 Aug;103(4):1970–1972. doi: 10.1111/j.1476-5381.1991.tb12361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pelayo F., Dubocovich M. L., Langer S. Z. Possible role of cyclic nucleotides in regulation of noradrenaline release from rat pineal through presynaptic adrenoceptors. Nature. 1978 Jul 6;274(5666):76–78. doi: 10.1038/274076a0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokas E. G., Folco G. C. Intima-related vasodilatation of the perfused rat caudal artery. Eur J Pharmacol. 1984 Apr 20;100(2):211–217. doi: 10.1016/0014-2999(84)90225-5. [DOI] [PubMed] [Google Scholar]
- Starke K. Presynaptic alpha-autoreceptors. Rev Physiol Biochem Pharmacol. 1987;107:73–146. [PubMed] [Google Scholar]
- Tesfamariam B., Weisbrod R. M., Cohen R. A. Endothelium inhibits responses of rabbit carotid artery to adrenergic nerve stimulation. Am J Physiol. 1987 Oct;253(4 Pt 2):H792–H798. doi: 10.1152/ajpheart.1987.253.4.H792. [DOI] [PubMed] [Google Scholar]
- Toda N., Baba H., Okamura T. Role of nitric oxide in non-adrenergic, non-cholinergic nerve-mediated relaxation in dog duodenal longitudinal muscle strips. Jpn J Pharmacol. 1990 Jun;53(2):281–284. doi: 10.1254/jjp.53.281. [DOI] [PubMed] [Google Scholar]
- Toda N., Minami Y., Okamura T. Inhibitory effects of L-NG-nitro-arginine on the synthesis of EDRF and the cerebroarterial response to vasodilator nerve stimulation. Life Sci. 1990;47(4):345–351. doi: 10.1016/0024-3205(90)90593-g. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Possible role of nitric oxide in transmitting information from vasodilator nerve to cerebroarterial muscle. Biochem Biophys Res Commun. 1990 Jul 16;170(1):308–313. doi: 10.1016/0006-291x(90)91275-w. [DOI] [PubMed] [Google Scholar]
- Toda N., Yoshida K., Okamura T. Analysis of the potentiating action of N(G)-nitro-L-arginine on the contraction of the dog temporal artery elicited by transmural stimulation of noradrenergic nerves. Naunyn Schmiedebergs Arch Pharmacol. 1991 Feb;343(2):221–224. doi: 10.1007/BF00168614. [DOI] [PubMed] [Google Scholar]
- Tucker J. F., Brave S. R., Charalambous L., Hobbs A. J., Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990 Aug;100(4):663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo P. A., Reid J. J., Rand M. J. Endothelial nitric oxide attenuates vasoconstrictor responses to nerve stimulation and noradrenaline in the rat tail artery. Eur J Pharmacol. 1991 Jun 18;199(1):123–125. doi: 10.1016/0014-2999(91)90647-9. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wennmalm A., Benthin G., Karwatowska-Prokopczuk E., Lundberg J., Petersson A. S. Release of endothelial mediators and sympathetic transmitters at different coronary flow rates in rabbit hearts. J Physiol. 1991 Apr;435:163–173. doi: 10.1113/jphysiol.1991.sp018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L., Corbin J. D., Francis S. H. Characterization of a novel isozyme of cGMP-dependent protein kinase from bovine aorta. J Biol Chem. 1989 May 5;264(13):7734–7741. [PubMed] [Google Scholar]


