Abstract
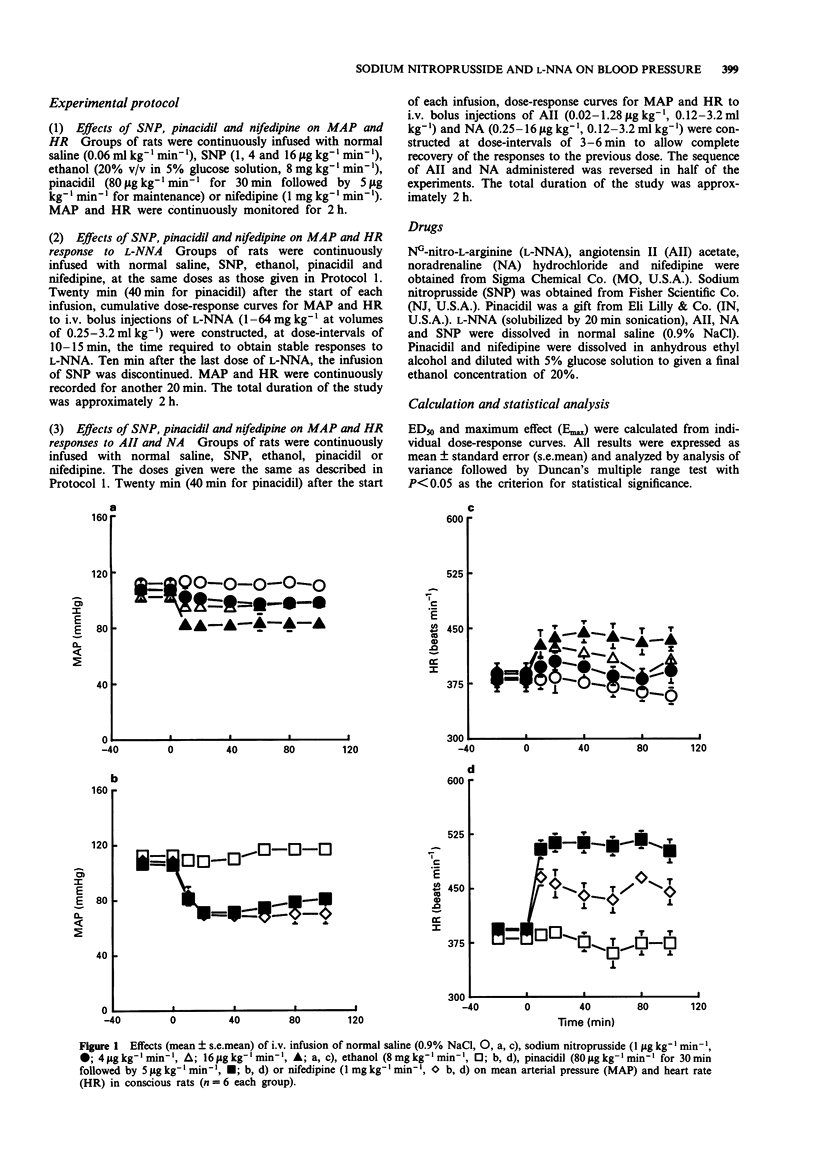
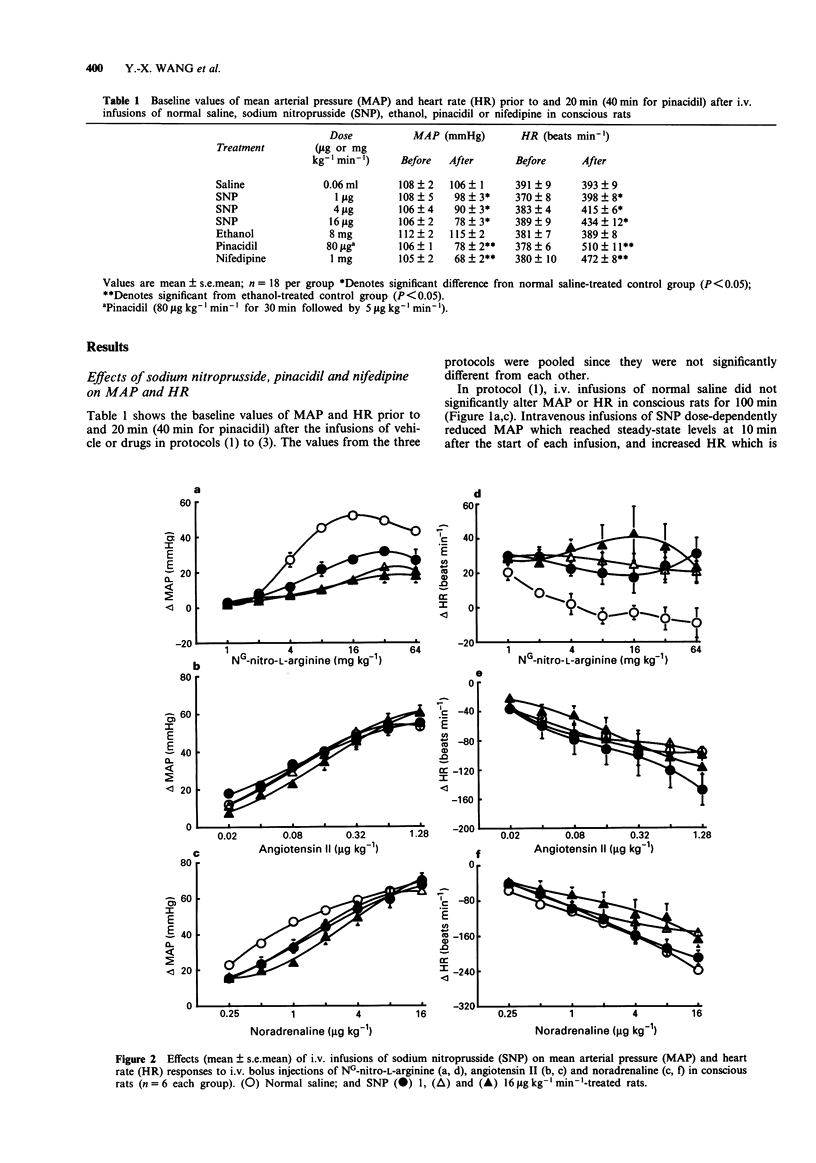
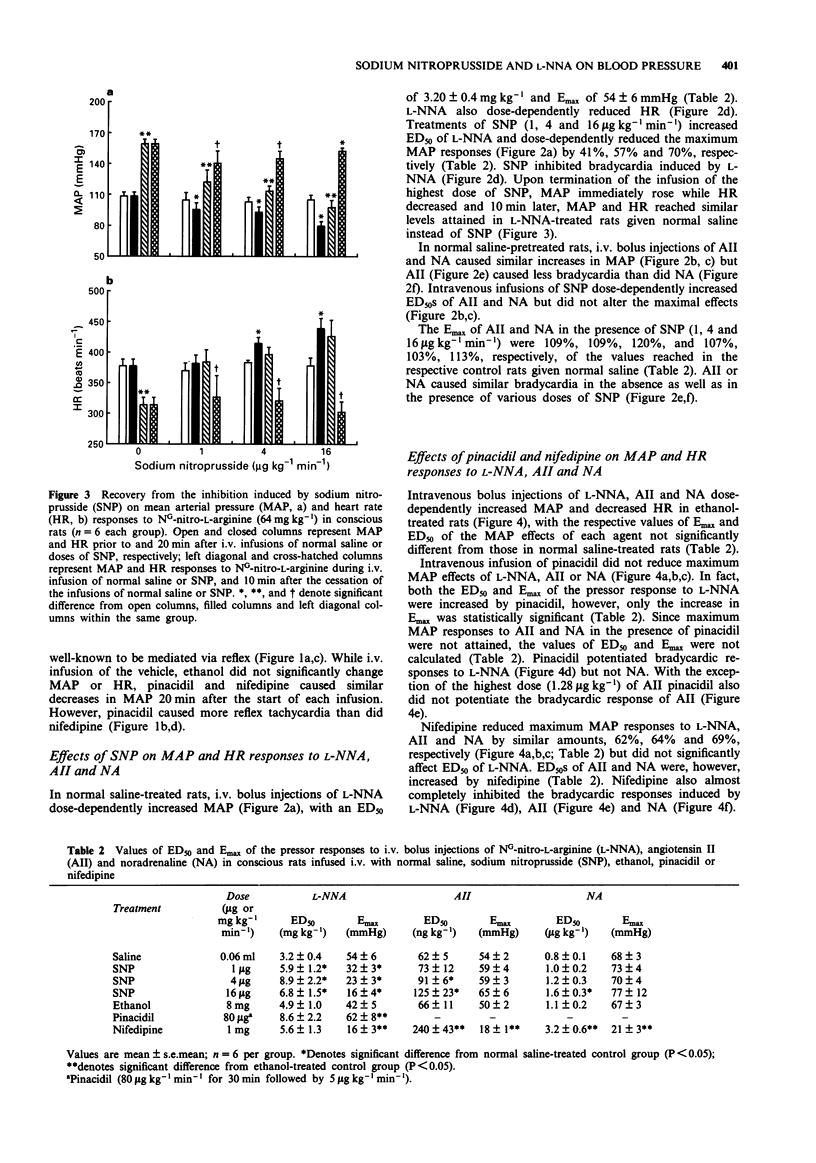
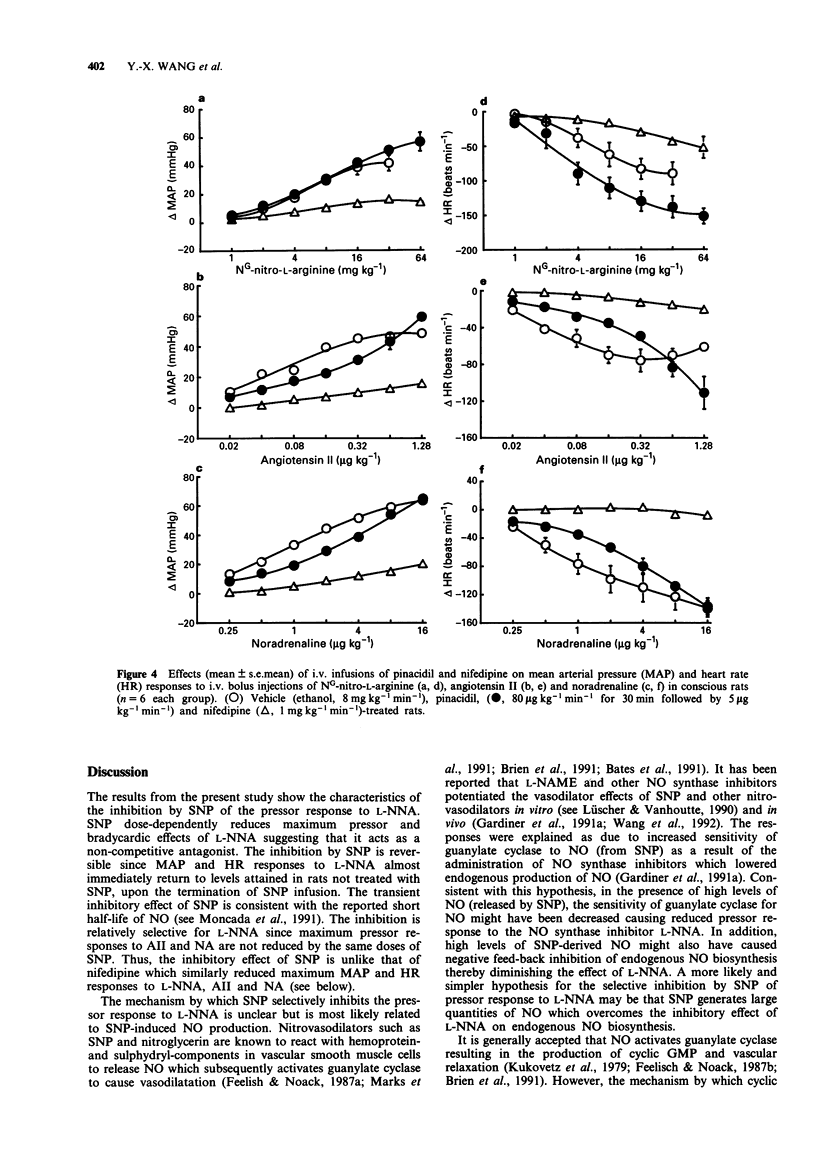
1. The inhibitory effects of sodium nitroprusside (SNP), a nitric oxide (NO) donor, on mean arterial pressure (MAP) responses to NG-nitro-L-arginine (L-NNA) (NO synthase inhibitor), angiotensin II (AII) and noradrenaline (NA) were compared with those of pinacidil (KATP channel opener) and nifedipine (L-type calcium antagonist) in conscious, unrestrained rats. 2. Intravenous bolus injections of L-NNA (1-64 mg kg-1), AII (0.02-1.28 micrograms kg-1) and NA (0.25-16 micrograms kg-1) dose-dependently increased MAP to similar maxima. Intravenous infusions of SNP (1, 4 and 16 micrograms kg-1 min-1) dose-dependently increased ED20S of L-NNA, AII and NA. However, the maximum response evoked by L-NNA, but not by AII nor NA, was dose-dependently reduced by SNP. Moreover, the inhibitory effect of SNP on the pressor response to L-NNA ceased when the infusion of SNP was terminated. 3. Pinacidil (80 micrograms kg-1 min-1 for 30 min followed by 5 micrograms kg-1 min-1) increased ED50S of L-NNA, AII and NA but did not decrease the maximum responses to any of these agents. 4. Nifedipine (1 mg kg-1 min-1) non-selectively reduced maximum responses to L-NNA, AII and NA to similar levels and increased ED50S of AII and NA but not L-NNA. 5. The results show that SNP causes a selective, non-competitive and reversible inhibition of the pressor response to L-NNA. This inhibition by SNP is unlikely to be related to hypotension, the opening of ATP-sensitive potassium channels or blockade of L-type calcium channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates J. N., Baker M. T., Guerra R., Jr, Harrison D. G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Bellan J. A., Minkes R. K., McNamara D. B., Kadowitz P. J. N omega-nitro-L-arginine selectively inhibits vasodilator responses to acetylcholine and bradykinin in cats. Am J Physiol. 1991 Mar;260(3 Pt 2):H1025–H1029. doi: 10.1152/ajpheart.1991.260.3.H1025. [DOI] [PubMed] [Google Scholar]
- Brien J. F., McLaughlin B. E., Nakatsu K., Marks G. S. Quantitation of nitric oxide formation from nitrovasodilator drugs by chemiluminescence analysis of headspace gas. J Pharmacol Methods. 1991 Mar;25(1):19–27. doi: 10.1016/0160-5402(91)90019-2. [DOI] [PubMed] [Google Scholar]
- Buckingham R. E. Studies on the anti-vasoconstrictor activity of BRL 34915 in spontaneously hypertensive rats; a comparison with nifedipine. Br J Pharmacol. 1988 Mar;93(3):541–552. doi: 10.1111/j.1476-5381.1988.tb10309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W., MacKay M. J. The effects of sodium nitroprusside and 8-bromo-cyclic GMP on electrical and mechanical activities of the rat tail artery. Br J Pharmacol. 1985 Sep;86(1):117–124. doi: 10.1111/j.1476-5381.1985.tb09441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Griffith T. M., Henderson A. H., Lewis M. J. Endothelium-derived relaxing factor alters calcium fluxes in rabbit aorta: a cyclic guanosine monophosphate-mediated effect. J Physiol. 1986 Dec;381:427–437. doi: 10.1113/jphysiol.1986.sp016336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S., Quast U., Hof R. P., Baumlin Y., Pally C. Similarities in the mechanism of action of two new vasodilator drugs: pinacidil and BRL 34915. J Cardiovasc Pharmacol. 1988 Jan;11(1):90–99. doi: 10.1097/00005344-198801000-00014. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Noack E. Nitric oxide (NO) formation from nitrovasodilators occurs independently of hemoglobin or non-heme iron. Eur J Pharmacol. 1987 Oct 27;142(3):465–469. doi: 10.1016/0014-2999(87)90090-2. [DOI] [PubMed] [Google Scholar]
- Fineman J. R., Heymann M. A., Soifer S. J. N omega-nitro-L-arginine attenuates endothelium-dependent pulmonary vasodilation in lambs. Am J Physiol. 1991 Apr;260(4 Pt 2):H1299–H1306. doi: 10.1152/ajpheart.1991.260.4.H1299. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1990 Nov;101(3):625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Kemp P. A., Bennett T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to acetylcholine, 5'-N-ethylcarboxamidoadenosine or salbutamol in conscious rats. Br J Pharmacol. 1991 Jul;103(3):1725–1732. doi: 10.1111/j.1476-5381.1991.tb09854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Kemp P. A., Bennett T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to adrenaline or BRL 38227 in conscious rats. Br J Pharmacol. 1991 Nov;104(3):731–737. doi: 10.1111/j.1476-5381.1991.tb12496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrino M. G., Plant T. D., Henquin J. C. Effects of putative activators of K+ channels in mouse pancreatic beta-cells. Br J Pharmacol. 1989 Nov;98(3):957–965. doi: 10.1111/j.1476-5381.1989.tb14626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. G., Carr R. D., Nicol A. K., Tomlinson W., O'Connor S. E. Coronary vasoconstriction in the conscious rabbit following intravenous infusion of L-NG-nitro-arginine. Br J Pharmacol. 1991 Mar;102(3):565–566. doi: 10.1111/j.1476-5381.1991.tb12212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Chang B., Kerwin J. F., Jr, Huang Z. J., Murad F. N omega-nitro-L-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. Eur J Pharmacol. 1990 Feb 6;176(2):219–223. doi: 10.1016/0014-2999(90)90531-a. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Actions of nitroglycerine on the membrane and mechanical properties of smooth muscles of the coronary artery of the pig. Br J Pharmacol. 1980 Oct;70(2):197–204. doi: 10.1111/j.1476-5381.1980.tb07925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Suzuki H., Kuriyama H. Effects of sodium nitroprusside on smooth muscle cells of rabbit pulmonary artery and portal vein. J Pharmacol Exp Ther. 1978 Dec;207(3):1022–1031. [PubMed] [Google Scholar]
- Kobayashi Y., Hattori K. Nitroarginine inhibits endothelium-derived relaxation. Jpn J Pharmacol. 1990 Jan;52(1):167–169. doi: 10.1254/jjp.52.167. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S., Wurm A., Pöch G. Evidence for cyclic GMP-mediated relaxant effects of nitro-compounds in coronary smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):129–138. doi: 10.1007/BF00500277. [DOI] [PubMed] [Google Scholar]
- Lacolley P. J., Lewis S. J., Brody M. J. L-NG-nitro arginine produces an exaggerated hypertension in anesthetized SHR. Eur J Pharmacol. 1991 May 17;197(2-3):239–240. doi: 10.1016/0014-2999(91)90533-v. [DOI] [PubMed] [Google Scholar]
- Lacolley P. J., Lewis S. J., Brody M. J. Role of sympathetic nerve activity in the generation of vascular nitric oxide in urethane-anesthetized rats. Hypertension. 1991 Jun;17(6 Pt 2):881–887. doi: 10.1161/01.hyp.17.6.881. [DOI] [PubMed] [Google Scholar]
- Lebrun P., Fang Z. Y., Antoine M. H., Herchuelz A., Hermann M., Berkenboom G., Fontaine J. Hypoglycemic sulfonylureas antagonize the effects of cromakalim and pinacidil on 86Rb fluxes and contractile activity in the rat aorta. Pharmacology. 1990;41(1):36–48. doi: 10.1159/000138697. [DOI] [PubMed] [Google Scholar]
- Marks G. S., McLaughlin B. E., Brown L. B., Beaton D. E., Booth B. P., Nakatsu K., Brien J. F. Interaction of glyceryl trinitrate and sodium nitroprusside with bovine pulmonary vein homogenate and 10,000 x g supernatant: biotransformation and nitric oxide formation. Can J Physiol Pharmacol. 1991 Jun;69(6):889–892. doi: 10.1139/y91-135. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Ratz P. H., Gleason M. M., Flaim S. F. Simultaneous measurement of force and calcium uptake during acetylcholine-induced endothelium-dependent relaxation of rabbit thoracic aorta. Circ Res. 1987 Jan;60(1):31–38. doi: 10.1161/01.res.60.1.31. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Tohse N., Sperelakis N. cGMP inhibits the activity of single calcium channels in embryonic chick heart cells. Circ Res. 1991 Aug;69(2):325–331. doi: 10.1161/01.res.69.2.325. [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Pang C. C. Possible dependence of pressor and heart rate effects of NG-nitro-L-arginine on autonomic nerve activity. Br J Pharmacol. 1991 Aug;103(4):2004–2008. doi: 10.1111/j.1476-5381.1991.tb12367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. X., Pang C. C. Pressor effect of NG-nitro-L-arginine in pentobarbital-anesthetized rats. Life Sci. 1990;47(24):2217–2224. doi: 10.1016/0024-3205(90)90152-h. [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Zhou T., Chua T. C., Pang C. C. Effects of inhalation and intravenous anesthetic agents on pressor response to NG-nitro-L-arginine. Eur J Pharmacol. 1991 Jun 6;198(2-3):183–188. doi: 10.1016/0014-2999(91)90619-2. [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Zhou T., Pang C. C. Pressor effects of L and D enantiomers of NG-nitro-arginine in conscious rats are antagonized by L- but not D-arginine. Eur J Pharmacol. 1991 Jul 23;200(1):77–81. doi: 10.1016/0014-2999(91)90668-g. [DOI] [PubMed] [Google Scholar]
- Yang T., Tande P. M., Refsum H. Negative chronotropic effect of a novel class III antiarrhythmic drug, UK-68,798, devoid of beta-blocking action on isolated guinea-pig atria. Br J Pharmacol. 1991 Jun;103(2):1417–1420. doi: 10.1111/j.1476-5381.1991.tb09804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


