Abstract
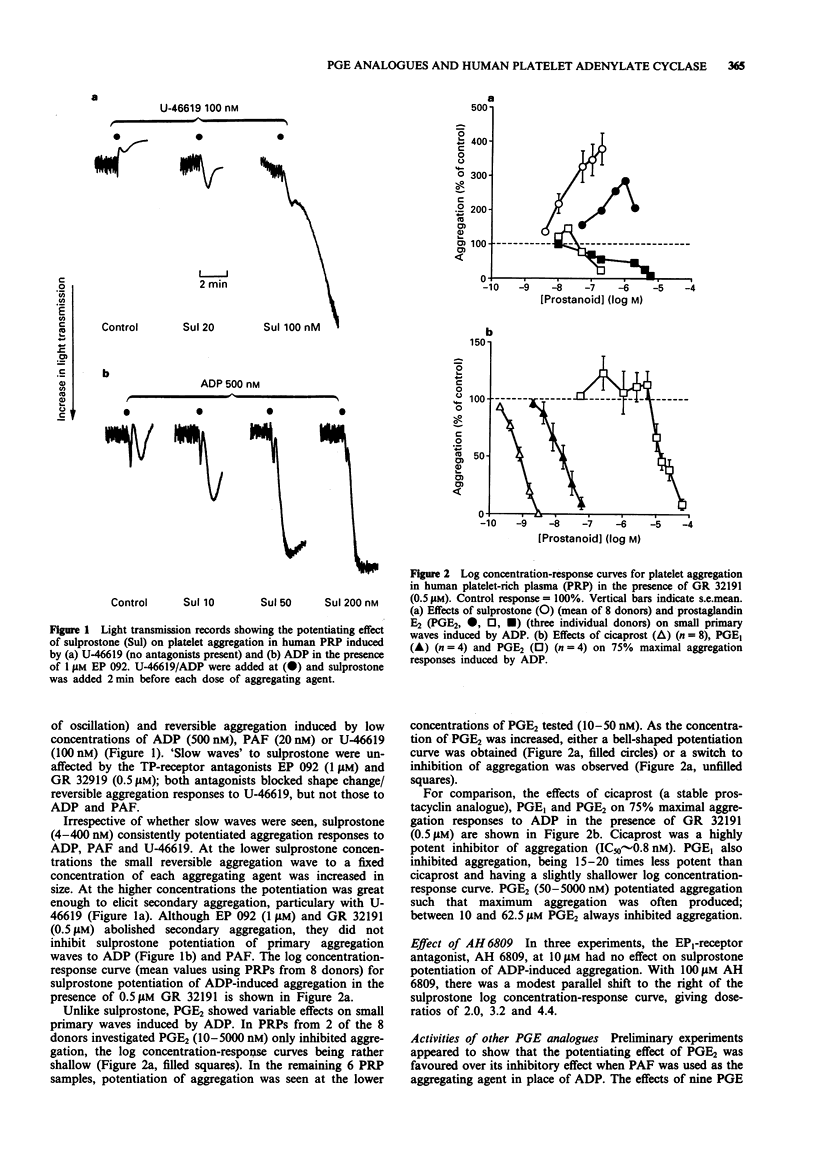
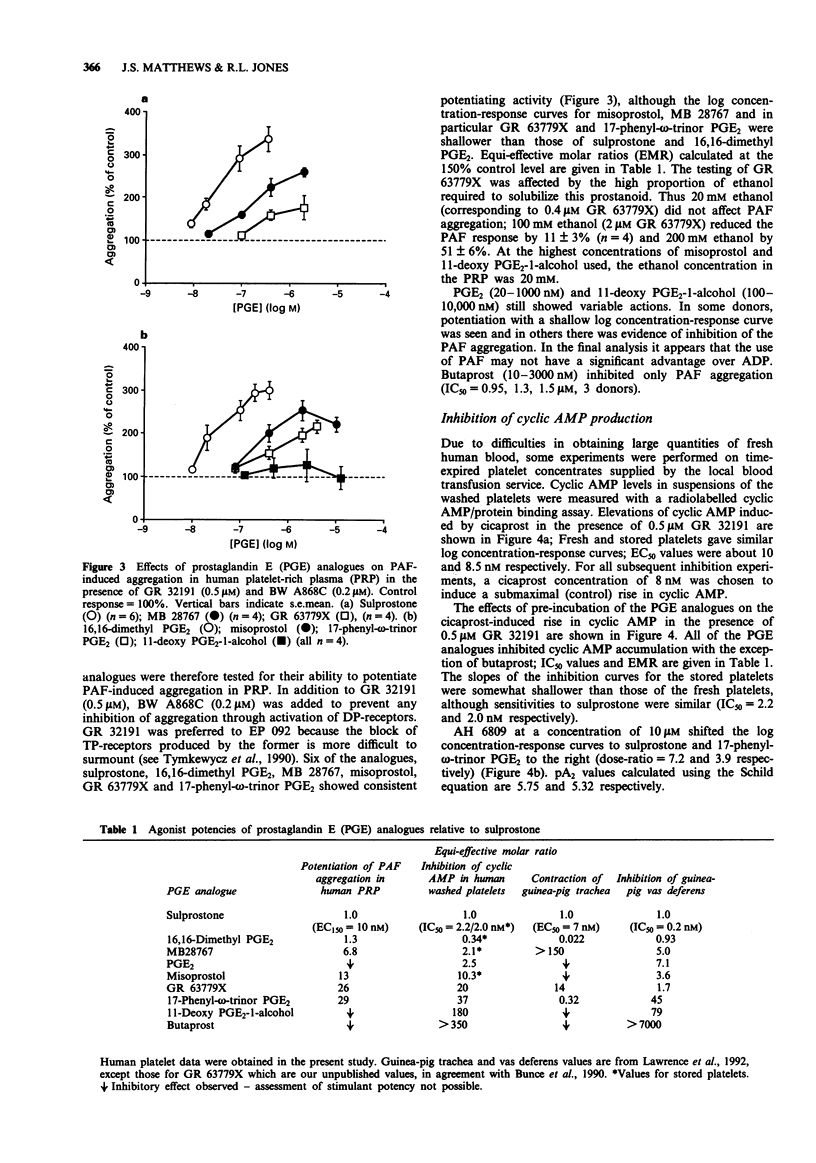
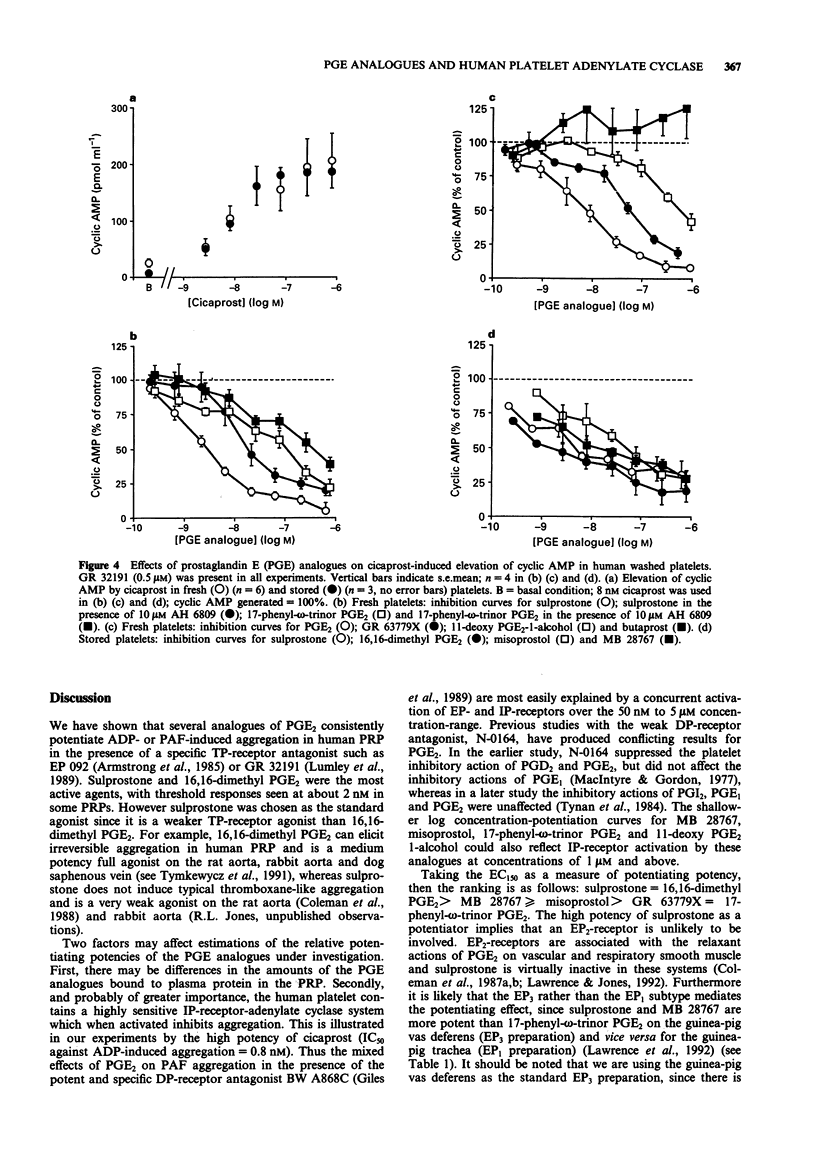
1. The 16-phenoxy prostaglandin E analogue sulprostone consistently potentiates primary aggregation waves induced by adenosine 5'-diphosphate (ADP), PAF and 11,9-epoxymethano PGH2 (U-46619) in platelet-rich plasma from human donors. The effect is not blocked by the TP-receptor antagonists, EP 092 and GR 32191. The high potency of sulprostone (threshold concentration = 4-10 nM) and the weak block of sulprostone potentiation by the EP1-receptor antagonist, AH 6809 (pA2 = 4.3) suggest the involvement of EP3-receptors as opposed to EP1- or EP2-subtypes. 2. Eight prostaglandin E (PGE) analogues were compared against sulprostone for their effects on PAF-induced aggregation in human platelet-rich plasma (PRP) in the presence of GR 32191 and the DP-receptor antagonist, BW A868C. PGE2 and 11-deoxy PGE2-1-alcohol showed evidence of both potentiating and inhibitory actions and butaprost showed only inhibitory activity at high concentrations. The remaining analogues always elicited potentiation, with the following potency ranking: sulprostone = 16,16-dimethyl PGE2 > MB 28767 > misoprostol > GR 63779X = 17-phenyl-omega-trinor PGE2. The results again indicate that EP3- rather than EP1- or EP2-receptors are involved. However, relative potentiating potency could be affected by differences in plasma protein binding and the very high sensitivity of the human platelet to prostacyclin (IP)-receptor-mediated inhibition (IC50 for the specific IP-receptor agonist cicaprost = 0.8 nM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen N. H., Eggerman T. L., Harker L. A., Wilson C. H., De B. On the multiplicity of platelet prostaglandin receptors. I. Evaluation of competitive antagonism by aggregometry. Prostaglandins. 1980 May;19(5):711–735. doi: 10.1016/0090-6980(80)90170-7. [DOI] [PubMed] [Google Scholar]
- Armstrong R. A., Jones R. L., Peesapati V., Will S. G., Wilson N. H. Competitive antagonism at thromboxane receptors in human platelets. Br J Pharmacol. 1985 Mar;84(3):595–607. doi: 10.1111/j.1476-5381.1985.tb16139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby B. Comparison of Iloprost, Cicaprost and prostacyclin effects on cyclic AMP metabolism in intact platelets. Prostaglandins. 1992 Mar;43(3):255–261. doi: 10.1016/0090-6980(92)90093-9. [DOI] [PubMed] [Google Scholar]
- Ashby B. Cyclic AMP turnover in response to prostaglandins in intact platelets: evidence for separate stimulatory and inhibitory prostaglandin receptors. Second Messengers Phosphoproteins. 1988;12(1):45–57. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Woolkalis M. J., Manning D. R. Interactions in platelets between G proteins and the agonists that stimulate phospholipase C and inhibit adenylyl cyclase. J Biol Chem. 1988 Apr 15;263(11):5348–5355. [PubMed] [Google Scholar]
- Dong Y. J., Jones R. L., Wilson N. H. Prostaglandin E receptor subtypes in smooth muscle: agonist activities of stable prostacyclin analogues. Br J Pharmacol. 1986 Jan;87(1):97–107. doi: 10.1111/j.1476-5381.1986.tb10161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerman T. L., Andersen N. H., Robertson R. P. Separate receptors for prostacyclin and prostaglandin E2 on human gel-filtered platelets. J Pharmacol Exp Ther. 1986 Mar;236(3):568–573. [PubMed] [Google Scholar]
- Giles H., Leff P., Bolofo M. L., Kelly M. G., Robertson A. D. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol. 1989 Feb;96(2):291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S. J., Heptinstall S. The effects of PGE2 and CL 115,347, an antihypertensive PGE2 analogue, on human blood platelet behaviour and vascular contractility. Eur J Pharmacol. 1985 Aug 15;114(2):129–137. doi: 10.1016/0014-2999(85)90620-x. [DOI] [PubMed] [Google Scholar]
- Gresele P., Blockmans D., Deckmyn H., Vermylen J. Adenylate cyclase activation determines the effect of thromboxane synthase inhibitors on platelet aggregation in vitro. Comparison of platelets from responders and nonresponders. J Pharmacol Exp Ther. 1988 Jul;246(1):301–307. [PubMed] [Google Scholar]
- Hourani S. M., Cusack N. J. Pharmacological receptors on blood platelets. Pharmacol Rev. 1991 Sep;43(3):243–298. [PubMed] [Google Scholar]
- Jones R. L., Peesapati V., Wilson N. H. Antagonism of the thromboxane-sensitive contractile systems of the rabbit aorta, dog saphenous vein and guinea-pig trachea. Br J Pharmacol. 1982 Jul;76(3):423–438. doi: 10.1111/j.1476-5381.1982.tb09236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keery R. J., Lumley P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br J Pharmacol. 1988 Jul;94(3):745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. A., Jones R. L. Investigation of the prostaglandin E (EP-) receptor subtype mediating relaxation of the rabbit jugular vein. Br J Pharmacol. 1992 Apr;105(4):817–824. doi: 10.1111/j.1476-5381.1992.tb09063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. A., Jones R. L., Wilson N. H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br J Pharmacol. 1992 Feb;105(2):271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley P., White B. P., Humphrey P. P. GR32191, a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle in vitro. Br J Pharmacol. 1989 Jul;97(3):783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre D. E., Gordon J. L. Discrimination between platelet prostaglandin receptors with a specific antagonist of bisenoic prostaglandins. Thromb Res. 1977 Dec;11(6):705–713. doi: 10.1016/0049-3848(77)90099-8. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., Salzman E. W., Gordon J. L. Prostaglandin receptors on human platelets. Structure-activity relationships of stimulatory prostaglandins. Biochem J. 1978 Sep 15;174(3):921–929. doi: 10.1042/bj1740921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. W., Stuart R. K. Interaction of prostaglandins E1 and E2 in regulation of cyclic-AMP and aggregation in human platelets: evidence for a common prostaglandin receptor. J Lab Clin Med. 1974 Jul;84(1):111–121. [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Shio H., Ramwell P. Effect of prostaglandin E 2 and aspirin on the secondary aggregation of human platelets. Nat New Biol. 1972 Mar 15;236(63):45–46. doi: 10.1038/newbio236045a0. [DOI] [PubMed] [Google Scholar]
- Siegl A. M., Smith J. B., Silver M. J., Nicolaou K. C., Ahern D. Selective binding site for [3H]prostacyclin on platelets. J Clin Invest. 1979 Feb;63(2):215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymkewycz P. M., Jones R. L., Wilson N. H., Marr C. G. Heterogeneity of thromboxane A2 (TP-) receptors: evidence from antagonist but not agonist potency measurements. Br J Pharmacol. 1991 Mar;102(3):607–614. doi: 10.1111/j.1476-5381.1991.tb12220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan S. S., Andersen N. H., Wills M. T., Harker L. A., Hanson S. R. On the multiplicity of platelet prostaglandin receptors. II. The use of N-0164 for distinguishing the loci of action for PGI2, PGD2, PGE2 and hydantoin analogs. Prostaglandins. 1984 May;27(5):683–696. doi: 10.1016/0090-6980(84)90007-8. [DOI] [PubMed] [Google Scholar]
- Vigdahl R. L., Marquis N. R., Tavormina P. A. Platelet aggregation. II. Adenyl cyclase, prostaglandin E1, and calcium. Biochem Biophys Res Commun. 1969 Oct 22;37(3):409–415. doi: 10.1016/0006-291x(69)90930-9. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Moncada S., Vane J. R. Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and D2 on platelet aggregation in different species. Prostaglandins. 1978 Sep;16(3):373–388. doi: 10.1016/0090-6980(78)90216-2. [DOI] [PubMed] [Google Scholar]



