Abstract
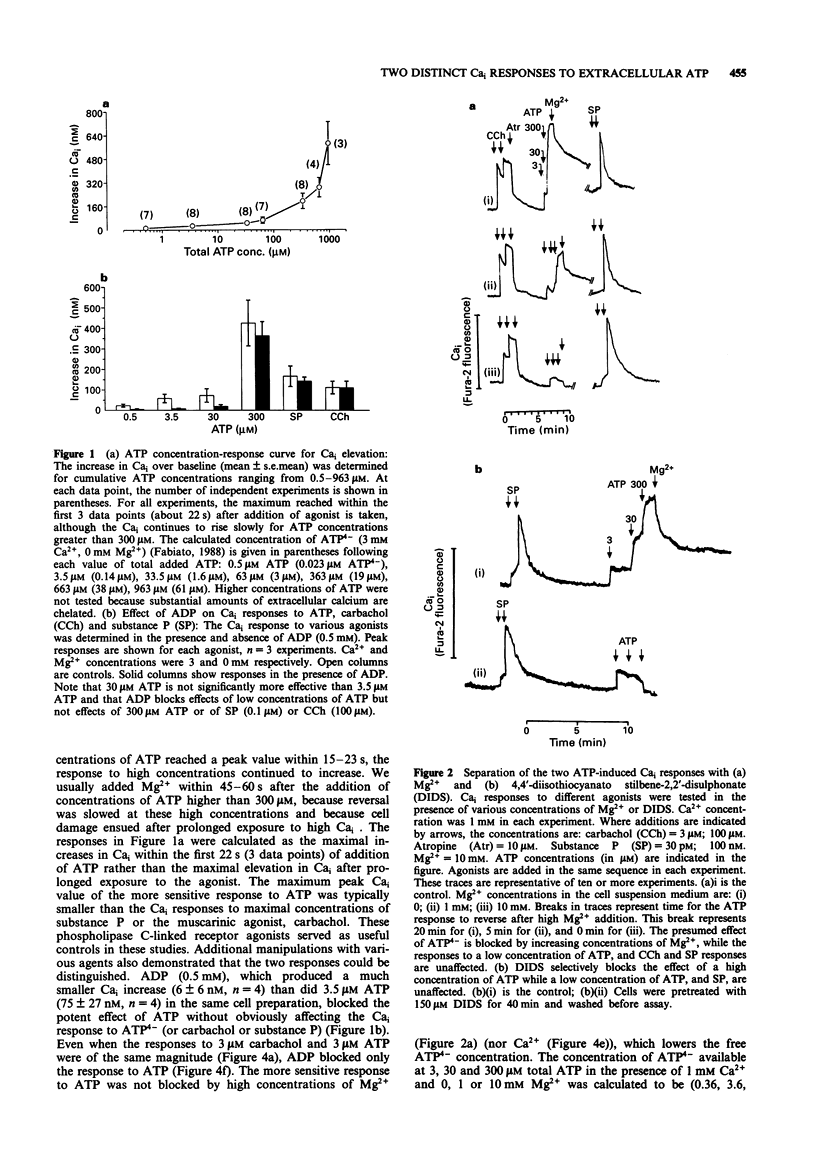
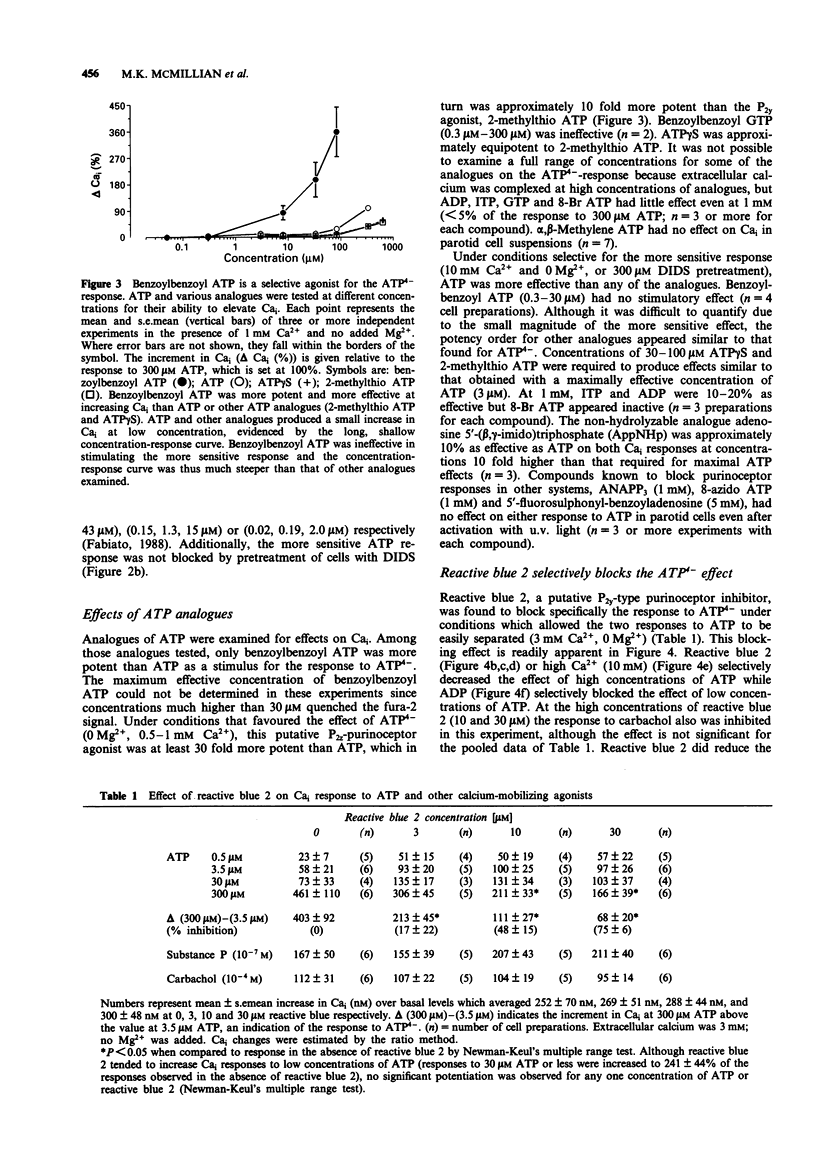
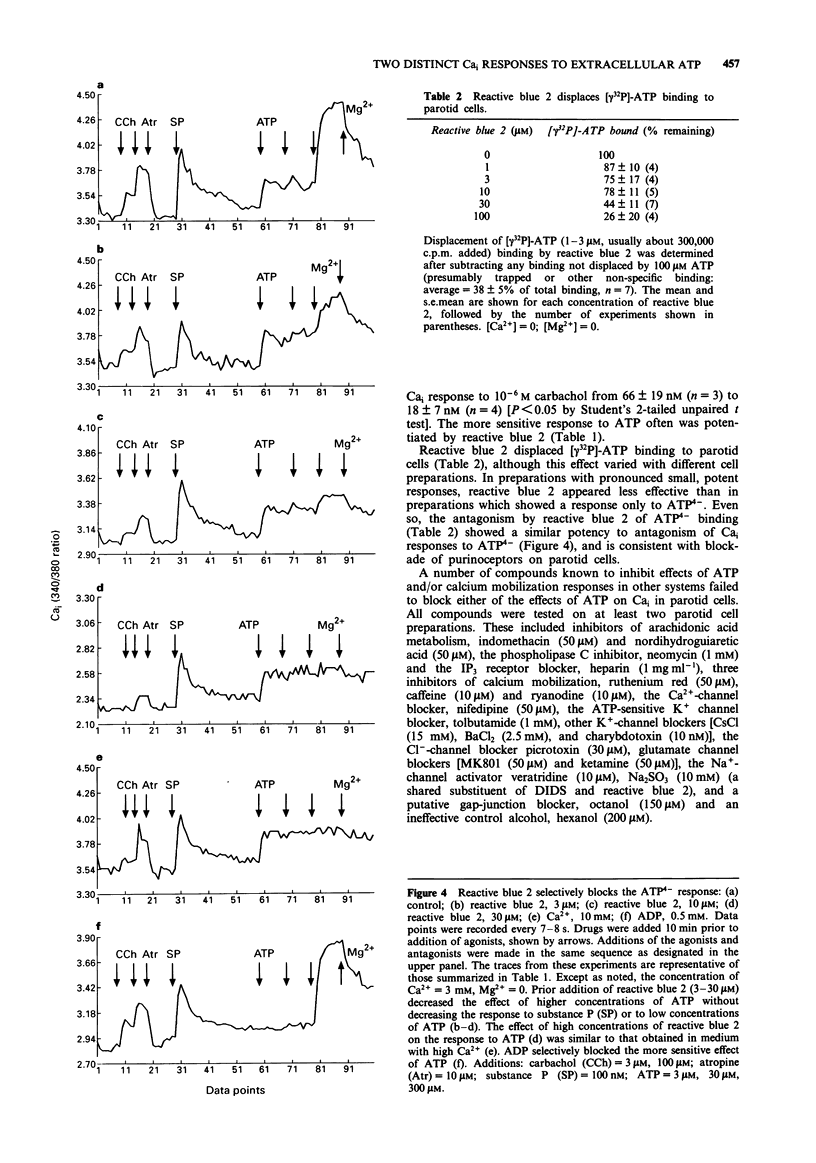
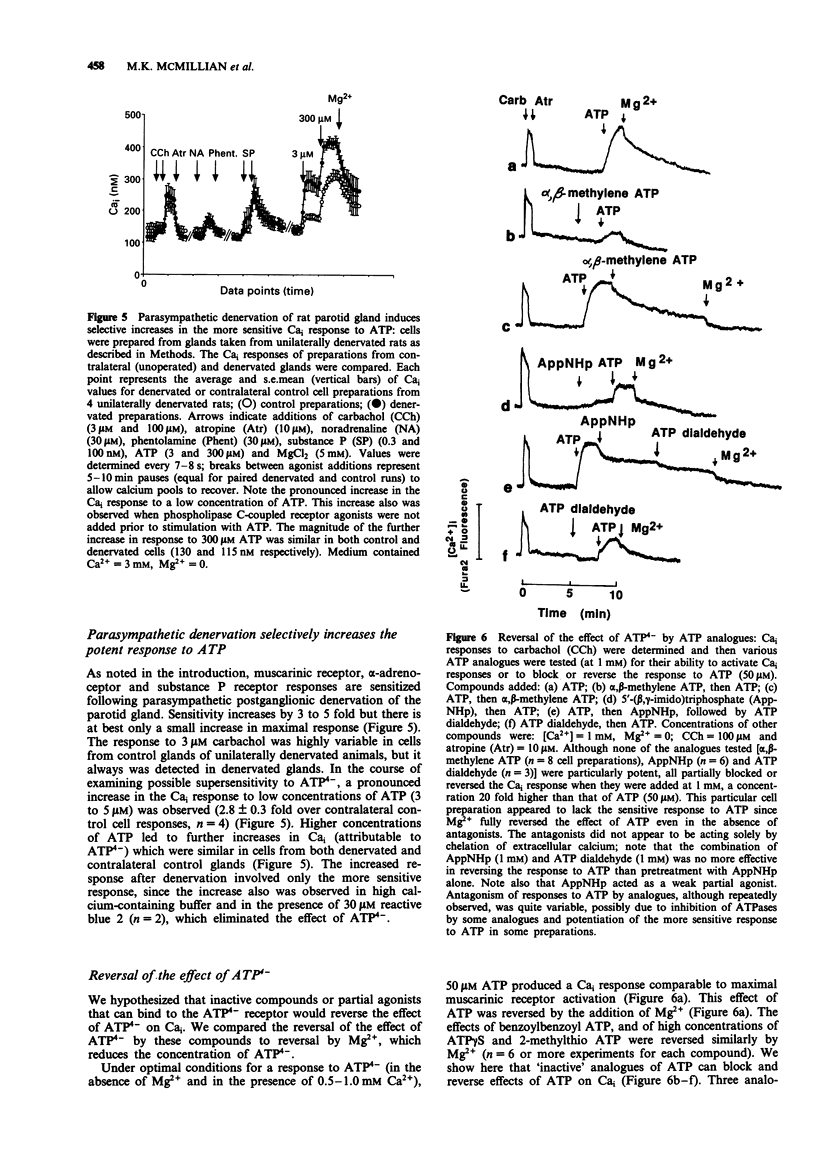
1. Increasing concentrations of ATP (0.5 microM-300 microM) produced a biphasic increase in intracellular calcium concentration [Ca]i in rat parotid acinar cells, reflecting two distinct Cai responses to extracellular ATP. 2. In the absence of Mg2+ (with 3 mM CaCl2 in the buffer solution), the more sensitive response was maximal at 3-5 microM and was not further increased by 30 microM ATP. This response to ATP was not well maintained and was blocked by ADP (0.5 mM). A second, much larger increase in Cai was observed on addition of 300 microM ATP. This larger effect, which we have described previously, appears to be mediated by ATP4-, and was selectively reversed by 4,4'-di-isothiocyanato-dihydrostilbene-2,2'-disulphonate as well as by high concentrations of alpha,beta-methylene ATP. 3. Among ATP analogues, only the putative P2Z agonist, 3'-0-(4-benzoyl)benzoyl-ATP distinguished between the two responses. This analogue was at least 10 fold more potent than ATP in stimulating the ATP(4-)-response, but did not evoke the more sensitive response. The agonist potency series for both responses to ATP was identical for other analogues examined (ATP > ATP gamma S = 2-methylthio ATP (a P2y-selective agonist) >> ADP, ITP and alpha,beta-methylene ATP (a P2x-selective agonist)). 4. Although the effect of ATP4- could best be characterized as a P2z-type purinoceptor response, this effect was strongly and selectively blocked by reactive blue 2, a putative P2y-purinoceptor antagonist. Reactive blue 2 may bind to and block P2z purinoceptors since [gamma 32P]-ATP binding to parotid cells was inhibited by this compound.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Downes C. P., Harden T. K. Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem. 1989 Jan 15;264(2):884–890. [PubMed] [Google Scholar]
- Boyer J. L., Harden T. K. Irreversible activation of phospholipase C-coupled P2Y-purinergic receptors by 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate. Mol Pharmacol. 1989 Dec;36(6):831–835. [PubMed] [Google Scholar]
- Buisman H. P., Steinberg T. H., Fischbarg J., Silverstein S. C., Vogelzang S. A., Ince C., Ypey D. L., Leijh P. C. Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7988–7992. doi: 10.1073/pnas.85.21.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purines and cotransmitters in adrenergic and cholinergic neurones. Prog Brain Res. 1986;68:193–203. doi: 10.1016/s0079-6123(08)60239-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo L. K. The effect of reactive blue, an antagonist of ATP, on the isolated urinary bladders of guinea-pig and rat. J Pharm Pharmacol. 1981 Apr;33(4):248–250. doi: 10.1111/j.2042-7158.1981.tb13770.x. [DOI] [PubMed] [Google Scholar]
- Cobbold P., Woods N., Wainwright J., Cuthbertson R. Single cell measurements in research on calcium-mobilising purinoceptors. J Recept Res. 1988;8(1-4):481–491. doi: 10.3109/10799898809049006. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. The ATP4- receptor of rat mast cells. Biochem J. 1980 Jun 15;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diverse-Pierluissi M., Dunlap K., Westhead E. W. Multiple actions of extracellular ATP on calcium currents in cultured bovine chromaffin cells. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1261–1265. doi: 10.1073/pnas.88.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström J., Malmberg L. Preponderance for either alpha- or beta-adrenoceptor mediated sensitization in the rat submaxillary gland. Acta Physiol Scand. 1981 Sep;113(1):103–110. doi: 10.1111/j.1748-1716.1981.tb06868.x. [DOI] [PubMed] [Google Scholar]
- Ekström J. Sensitization of the rat parotid gland to secretagogues following either parasympathetic denervation or sympathetic denervation or decentralization. Acta Physiol Scand. 1980 Mar;108(3):253–261. doi: 10.1111/j.1748-1716.1980.tb06531.x. [DOI] [PubMed] [Google Scholar]
- Erb L., Lustig K. D., Ahmed A. H., Gonzalez F. A., Weisman G. A. Covalent incorporation of 3'-O-(4-benzoyl)benzoyl-ATP into a P2 purinoceptor in transformed mouse fibroblasts. J Biol Chem. 1990 May 5;265(13):7424–7431. [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Ahmed A. H., Lustig K. D., Erb L., Weisman G. A. Permeabilization of transformed mouse fibroblasts by 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate and the desensitization of the process. J Cell Physiol. 1989 Apr;139(1):109–115. doi: 10.1002/jcp.1041390116. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Houston D. A., Burnstock G., Vanhoutte P. M. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987 May;241(2):501–506. [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Ohara-Imaizumi M., Obama T., Fujimori K., Takanaka A. Antagonism by reactive blue 2 but not by brilliant blue G of extracellular ATP-evoked responses in PC12 phaeochromocytoma cells. Br J Pharmacol. 1991 Apr;102(4):851–854. doi: 10.1111/j.1476-5381.1991.tb12265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. D., Milani D., Dunne M. J., Pralong W. F., Theler J. M., Petersen O. H., Wollheim C. B. Extracellular ATP causes Ca2(+)-dependent and -independent insulin secretion in RINm5F cells. Phospholipase C mediates Ca2+ mobilization but not Ca2+ influx and membrane depolarization. J Biol Chem. 1991 Feb 25;266(6):3449–3457. [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Cantley L. C., Talamo B. R. Extracellular ATP elevates intracellular free calcium in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):523–530. doi: 10.1016/0006-291x(87)90399-8. [DOI] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Lechleiter J. D., Cantley L. C., Talamo B. R. Extracellular ATP increases free cytosolic calcium in rat parotid acinar cells. Differences from phospholipase C-linked receptor agonists. Biochem J. 1988 Oct 1;255(1):291–300. [PMC free article] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Talamo B. R. Rapid desensitization of substance P- but not carbachol-induced increases in inositol trisphosphate and intracellular Ca++ in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1017–1024. doi: 10.1016/s0006-291x(87)80233-4. [DOI] [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Reilly W. M., Saville V. L., Burnstock G. An assessment of the antagonistic activity of reactive blue 2 at P1- and P2-purinoceptors: supporting evidence for purinergic innervation of the rabbit portal vein. Eur J Pharmacol. 1987 Aug 4;140(1):47–53. doi: 10.1016/0014-2999(87)90632-7. [DOI] [PubMed] [Google Scholar]
- Richards N. W., Allbee W. E., Gaginella T. S., Wallace L. J. Exogenous ATP-stimulated calcium uptake in isolated rat intestinal epithelial cells. Life Sci. 1987 Apr 27;40(17):1665–1672. doi: 10.1016/0024-3205(87)90015-4. [DOI] [PubMed] [Google Scholar]
- Rohde G. C., Huidobro-Toro J. P. Purinergic supersensitivity following sympathectomy adds further support to co-transmission in the rat vas deferens. Arch Int Pharmacodyn Ther. 1988 Jul-Aug;294:99–111. [PubMed] [Google Scholar]
- Sasaki T., Gallacher D. V. Extracellular ATP activates receptor-operated cation channels in mouse lacrimal acinar cells to promote calcium influx in the absence of phosphoinositide metabolism. FEBS Lett. 1990 May 7;264(1):130–134. doi: 10.1016/0014-5793(90)80782-e. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Cragoe E. J., Jr, Cantley L. C., Talamo B. R. Effects of extracellular ATP on ion transport systems and [Ca2+]i in rat parotid acinar cells. Comparison with the muscarinic agonist carbachol. J Gen Physiol. 1990 Feb;95(2):319–346. doi: 10.1085/jgp.95.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Talamo B. R. ATP activates a cation-permeable pathway in rat parotid acinar cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C934–C940. doi: 10.1152/ajpcell.1992.262.4.C934. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Talamo B. R. Coomassie Brilliant Blue G is a more potent antagonist of P2 purinergic responses than Reactive Blue 2 (Cibacron Blue 3GA) in rat parotid acinar cells. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1279–1285. doi: 10.1016/0006-291x(89)92741-1. [DOI] [PubMed] [Google Scholar]
- Talamo B. R., Adler S. C., Burt D. R. Parasympathetic denervation decreases muscarinic receptor binding in rat parotid. Life Sci. 1979 Apr 23;24(17):1573–1580. doi: 10.1016/0024-3205(79)90018-3. [DOI] [PubMed] [Google Scholar]
- Tatham P. E., Cusack N. J., Gomperts B. D. Characterisation of the ATP4- receptor that mediates permeabilisation of rat mast cells. Eur J Pharmacol. 1988 Feb 16;147(1):13–21. doi: 10.1016/0014-2999(88)90628-0. [DOI] [PubMed] [Google Scholar]
- Weisman G. A., Dunn S. D., De B. K., Kitagawa T., Friedberg I. On the role of protein phosphorylation in the ATP-dependent permeabilization of transformed cells. J Cell Physiol. 1984 Feb;118(2):124–132. doi: 10.1002/jcp.1041180204. [DOI] [PubMed] [Google Scholar]
- Williams N., Coleman P. S. Exploring the adenine nucleotide binding sites on mitochondrial F1-ATPase with a new photoaffinity probe, 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate. J Biol Chem. 1982 Mar 25;257(6):2834–2841. [PubMed] [Google Scholar]
- el-Moatassim C., Maurice T., Mani J. C., Dornand J. The [Ca2+]i increase induced in murine thymocytes by extracellular ATP does not involve ATP hydrolysis and is not related to phosphoinositide metabolism. FEBS Lett. 1989 Jan 2;242(2):391–396. doi: 10.1016/0014-5793(89)80508-3. [DOI] [PubMed] [Google Scholar]


