Abstract
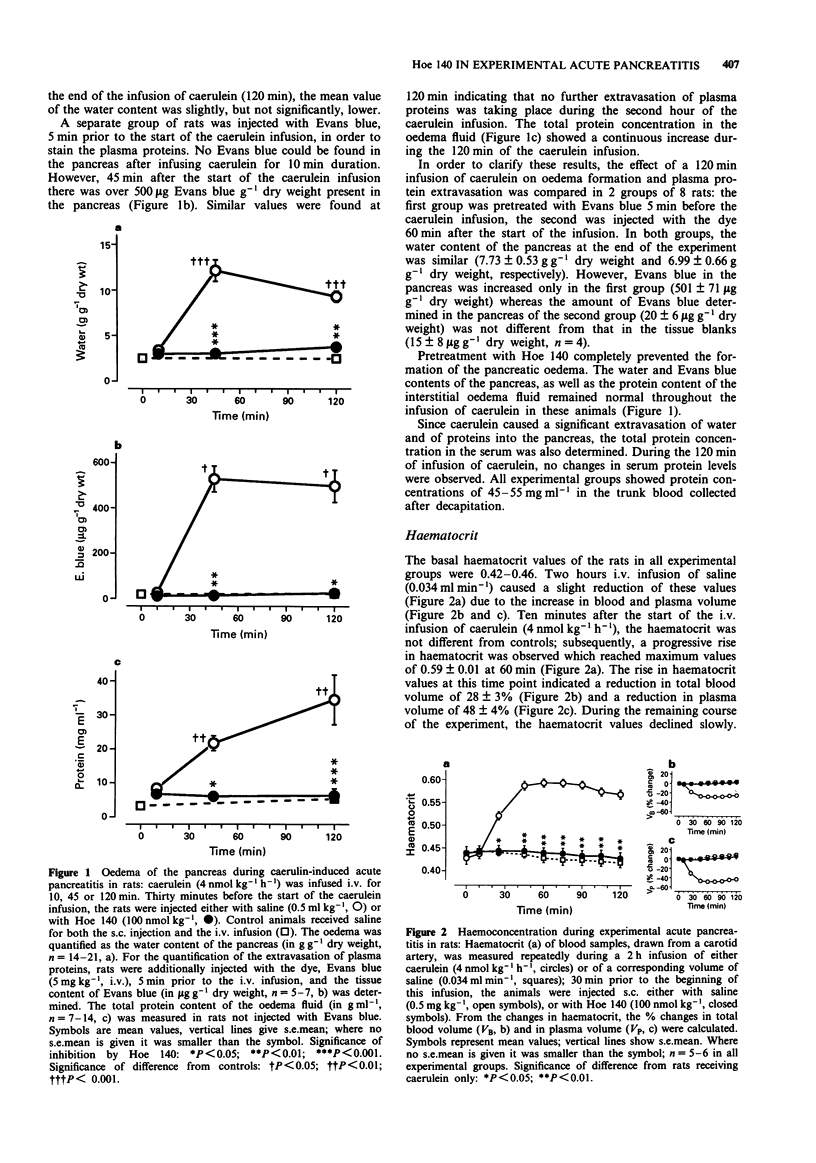
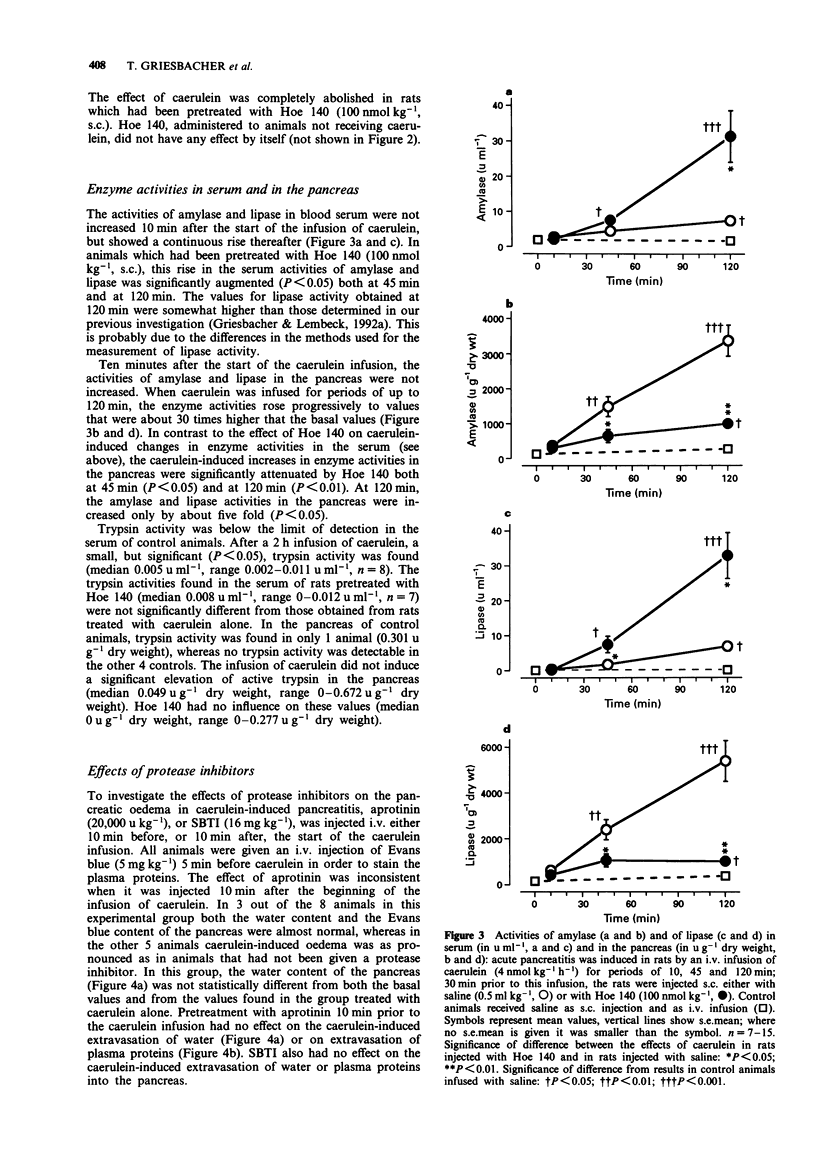
1. In a previous investigation, Hoe 140, a specific and potent bradykinin B2 receptor antagonist, prevented the pancreatic oedema and the hypotension observed during acute experimental pancreatitis; however, it augmented the associated rises in the serum activities of pancreatic enzymes. Therefore, we have now investigated the consequences of the pancreatic oedema for the fate of activated enzymes released into the tissue during the course of acute pancreatitis. 2. Acute oedematous pancreatitis was induced in rats, pretreated with captopril (50 mumol kg-1, i.p.), by hyperstimulation of the exocrine function of the pancreas with the cholecystokinin analogue, caerulein (4 nmol kg-1 h-1, i.v.), for up to 120 min. 3. Pancreatic oedema began to develop 10 min after the start of the caerulein infusion, reached a maximum within about 45 min, and then declined slightly. The development of the oedema parallelled the second phase of the caerulein-induced fall in blood pressure found in earlier experiments. No further extravasation of plasma proteins occurred during the 2nd hour of the caerulein infusion. The oedema formation was completely blocked in animals pretreated with the bradykinin receptor antagonist, Hoe 140 (100 nmol kg-1, s.c.). Pretreatment with aprotinin or soy bean trypsin inhibitor did not result in a significant inhibition of the oedema. 4. The haematocrit of animals with experimental pancreatitis showed a pronounced increase which started 10 min after the start of the caerulein infusion and reached maximal values at 60 min. The changes in haematocrit showed a reduction in total blood volume of 28% due to a 48% loss of plasma. This effect was completely blocked by Hoe 140.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
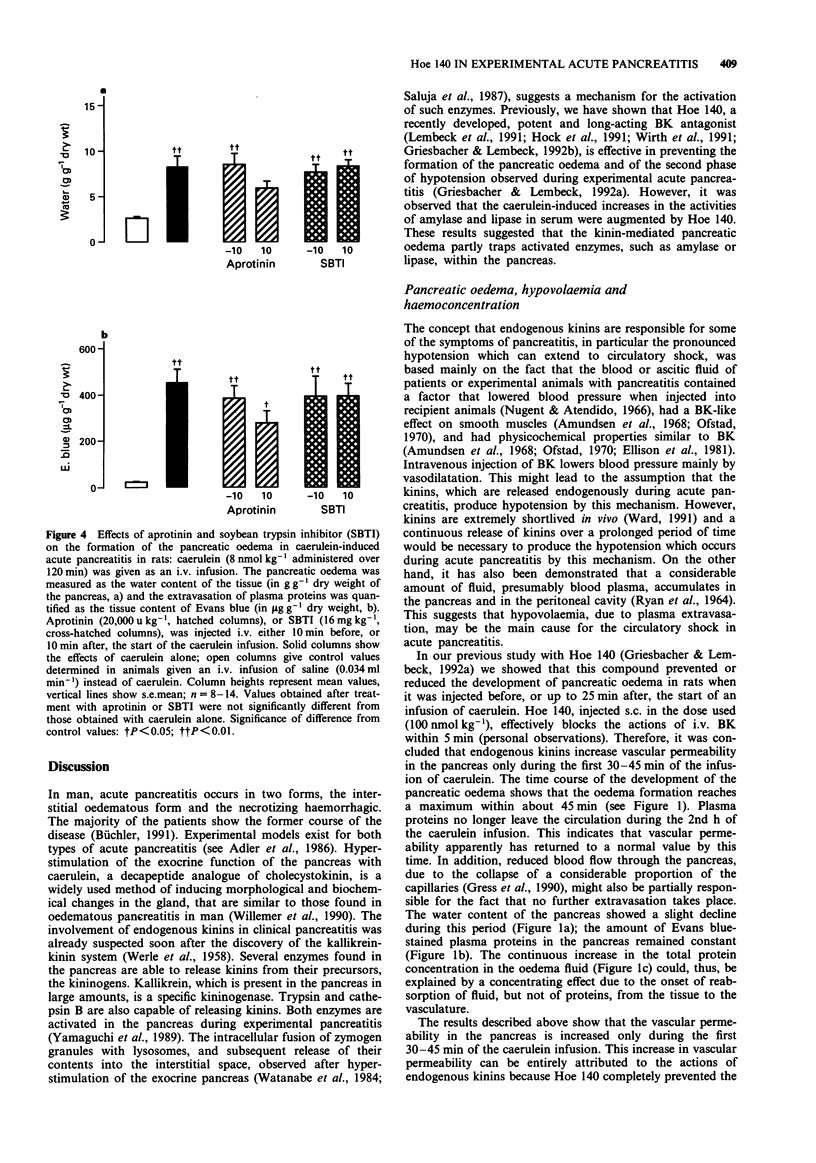
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amundsen E., Ofstad E., Hagen P. O. Experimental acute pancreatitis in dogs. I. Hypotensive effect induced by pancreatic exudate. Scand J Gastroenterol. 1968;3(6):659–664. [PubMed] [Google Scholar]
- Creutzfeldt W., Schmidt H. Aetiology and pathogenesis of pancreatitis. (Current concepts). Scand J Gastroenterol Suppl. 1970;6:47–62. [PubMed] [Google Scholar]
- Ellison E. C., Pappas T. N., Johnson J. A., Fabri P. J., Carey L. C. Demonstration and characterization of the hemoconcentrating effect of ascitic fluid that accumulates during hemorrhagic pancreatitis. J Surg Res. 1981 Mar;30(3):241–248. doi: 10.1016/0022-4804(81)90155-4. [DOI] [PubMed] [Google Scholar]
- FORELL M. M. Zur Frage des Entstehungsmechanismus des Kreislaufkollapses bei der akuten Pankreasnekrose. Gastroenterologia. 1955;84(4):225–251. [PubMed] [Google Scholar]
- Gamse R., Holzer P., Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980 Feb;68(2):207–213. doi: 10.1111/j.1476-5381.1980.tb10409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gress T. M., Arnold R., Adler G. Structural alterations of pancreatic microvasculature in cerulein-induced pancreatitis in the rat. Res Exp Med (Berl) 1990;190(6):401–412. doi: 10.1007/BF00000046. [DOI] [PubMed] [Google Scholar]
- Griesbacher T., Lembeck F. Analysis of the antagonistic actions of HOE 140 and other novel bradykinin analogues on the guinea-pig ileum. Eur J Pharmacol. 1992 Feb 18;211(3):393–398. doi: 10.1016/0014-2999(92)90397-m. [DOI] [PubMed] [Google Scholar]
- Griesbacher T., Lembeck F. Effects of the bradykinin antagonist, HOE 140, in experimental acute pancreatitis. Br J Pharmacol. 1992 Oct;107(2):356–360. doi: 10.1111/j.1476-5381.1992.tb12751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Miyake H., Ochi K., Tanaka J., Kimura I. Clinical trial with a protease inhibitor gabexate mesilate in acute pancreatitis. Int J Pancreatol. 1991 Summer;9:75–79. doi: 10.1007/BF02925581. [DOI] [PubMed] [Google Scholar]
- Hock F. J., Wirth K., Albus U., Linz W., Gerhards H. J., Wiemer G., Henke S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br J Pharmacol. 1991 Mar;102(3):769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembeck F., Griesbacher T., Eckhardt M., Henke S., Breipohl G., Knolle J. New, long-acting, potent bradykinin antagonists. Br J Pharmacol. 1991 Feb;102(2):297–304. doi: 10.1111/j.1476-5381.1991.tb12169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent F. W., Atendido W. Hemorrhagic pancreatitis. Aggressive treatment. Postgrad Med. 1966 Jul;40(1):87–94. doi: 10.1080/00325481.1966.11695873. [DOI] [PubMed] [Google Scholar]
- Ofstad E. Formation and destruction of plasma kinins during experimental acute hemorrhagic pancreatitis in dogs. Scand J Gastroenterol Suppl. 1970;5:1–44. [PubMed] [Google Scholar]
- RYAN J. W., MOFFAT J. G., THOMPSON A. G. ROLE OF BRADYKININ IN THE DEVELOPMENT OF ACUTE PANCREATITIS. Nature. 1964 Dec 19;204:1212–1213. doi: 10.1038/2041212a0. [DOI] [PubMed] [Google Scholar]
- Saluja A., Hashimoto S., Saluja M., Powers R. E., Meldolesi J., Steer M. L. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol. 1987 Oct;253(4 Pt 1):G508–G516. doi: 10.1152/ajpgi.1987.253.4.G508. [DOI] [PubMed] [Google Scholar]
- Saria A., Lundberg J. M. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Methods. 1983 May;8(1):41–49. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- Steinberg W. M., Schlesselman S. E. Treatment of acute pancreatitis. Comparison of animal and human studies. Gastroenterology. 1987 Dec;93(6):1420–1427. doi: 10.1016/0016-5085(87)90275-7. [DOI] [PubMed] [Google Scholar]
- Trapnell J. E., Rigby C. C., Talbot C. H., Duncan E. H. A controlled trial of Trasylol in the treatment of acute pancreatitis. Br J Surg. 1974 Mar;61(3):177–182. doi: 10.1002/bjs.1800610303. [DOI] [PubMed] [Google Scholar]
- WERLE E., TAUBER K., HARTENBACH W., FORELL M. M. Zur Frage der Pankreatitis. Munch Med Wochenschr. 1958 Aug 29;100(35):1265–1267. [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- Willemer S., Bialek R., Köhler H., Adler G. Caerulein-induced acute pancreatitis in rats: changes in glycoprotein-composition of subcellular membrane systems in acinar cells. Histochemistry. 1990;95(1):87–96. doi: 10.1007/BF00737232. [DOI] [PubMed] [Google Scholar]
- Wirth K., Hock F. J., Albus U., Linz W., Alpermann H. G., Anagnostopoulos H., Henk S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br J Pharmacol. 1991 Mar;102(3):774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff G. N., Hughes J. Cholecystokinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:469–501. doi: 10.1146/annurev.pa.31.040191.002345. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kimura T., Mimura K., Nawata H. Activation of proteases in cerulein-induced pancreatitis. Pancreas. 1989;4(5):565–571. doi: 10.1097/00006676-198910000-00007. [DOI] [PubMed] [Google Scholar]


